Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Research Article - (2021)Volume 10, Issue 7
Aim: To assess the role of color doppler and pulse wave doppler indices resistive index (RI) and Pulsatility index (PI) of testicular artery as a predictor for abnormal spermatogenesis and subfertility in male.
Patients and methods: This is a retrospective study done on 50 patients,in which RI and PI of intratesticular vessels of both sides were calculated by Colour Doppler Ultrasound(CDUS). Results were statistically analysed to find out the cut-off of RI and PI values for spermatogenesis to occur normally.Their relation with presence of varicocele and History of Abortion was also studied.
Results: Individuals with normal sperm counts had mean (SD) RI <0.62 mean (SD) PI <1.04. Those with abnormal pathological sperm counts had mean (SD) RI=>0.62 mean (SD) PI=>1.04
Conclusion: These results suggest that CDUS RI and PI parameter can be used as supportive predictor for spermatogenesis and infertility
Scrotal doppler; Male infertility; Abnormal spermatogenesis; Resistive index; Pulsatility index
Infertility is defined as couples inability to conceive following a year of unprotected sexual intercourse [1,2]. Infertility is a prevalent disorder among the reproductive aged couples that affects approximately 15% of them [3-6].
Male infertility, alone or in combination with female infertility, is attributed to 50% of all cases of infertility [7-10].
Spermatogenesis is the process by which male primary germ cells undergo division, and produce a number of cells termed spermatogonia, from which the primary spermatocytes are derived [11].
Each primary spermatocyte divides into two secondary spermatocytes, and each secondary spermatocyte into two spermatids or young spermatozoa. These develop into mature spermatozoa, also known as sperm cells. Thus, the primary spermatocyte gives rise to two cells, the secondary spermatocytes, and the two secondary spermatocytes by their subdivision produce four spermatozoa.
Spermatogenesis occurs in the male testes, blood supply to testes is derived from testicular arteries which arise from aorta on Right (Rt.) side directly and from renal artery on Left (Lt.) side ,other source of blood supply include the deferential artery which supply epididymis, vas deferens and the cremasteric artery. The primary investigation for male factor is semen analysis. Due to technological advances in ultrasonography, the role of sonography in evaluating male infertility is expanding [12].
Increased RI and PI of intratesticular arteries on colour doppler ultrasound examination may be an indicator of impaired testicular microcirculation and a cause of impaired semen parameters. Following decreased testicular arterial flow, impaired spermatogenesis may result due to defective energy metabolism in the microcirculatory bed [13].
Resistive Index (RI) and Pulsatility Index (PI) are ultrasonic parameters showing testicular parenchymal perfusion and microcirculation function. RI is measured using S-D/S formula, where S represents the peak systolic velocity (PSV) and D indicate the end diastolic velocity (EDV).
PI is measured as S-D/A A (Time averaged Velocity).The increase in these indices in the testicles indicates disruption in micro- circulation and thus a significant reduction in testicular blood flow . As spermatogenesis is a highly sensitive process where blood demand and supply are of special significance, changes in the blood flow of the area could lead to impairment in sperm production [14-17].
New studies with colour doppler ultrasonography show a correlation between RI of intratesticular arteries and testicular function [18-21]. In our study we have used PI also to find it’s correlation with semen abnormalities. Semen analysis has its own limitations. Also, many men are uncomfortable with masturbating for semen collection, especially when performed at clinical laboratory. Therefore developing and using more convenient and more accurate diagnostic modalities deserves further investigation [22-26]. Moreover, the diagnostic value of RI and PI by doppler ultrasonography, as a non-invasive investigative method, has not been well determined yet. The aim of this study was to evaluate the diagnostic accuracy of intratesticular RI and PI by CDUS to predict impaired spermatogenesis.
This prospective study includes 50 age-matched individuals, their ages range between 20-50 years. Patients included in this study are male partners of the couples presenting with infertility at Dr.Nagori Institute for Infertility and IVF Ahmedabad. Seminal fluid analysis was done according to WHO guidelines in 2010 using samples after 3 days of sexual abstinence. All patients are assessed clinically for history of cryptorchidism, genital tract infection, and trauma. Physical examination, seminal fluid analysis and scrotal CDUS examination was done after taking verbal consent from the patient. Patients with history of trauma to testicles, surgery or clinically detectable diseases where excluded from the study. Scrotal ultrasound and CDUS done in warm room with patient lying supine, penis resting over lower abdomen, doppler flow were measured in each testes using transscrotal approach with 11 mega-hertz linear array probe using testicular examination software available on Voluson E10 at Dr. Nagori Institute Ahmedabad. Doppler indices PI and RI were calculated and recorded for both testicles for each patient. Statistical analysis includes finding the cutoff of RI and PI values for individuals with normal and abnormal semen. Semen Analysis was considered abnormal if any parameter mentioned in the WHO 2010 criteria does not match the normal limits. Study was also done to find out any correlation between RI and PI values and History of Abortions as well as presence of varicocele on CDUS (Figures 1 and 2).
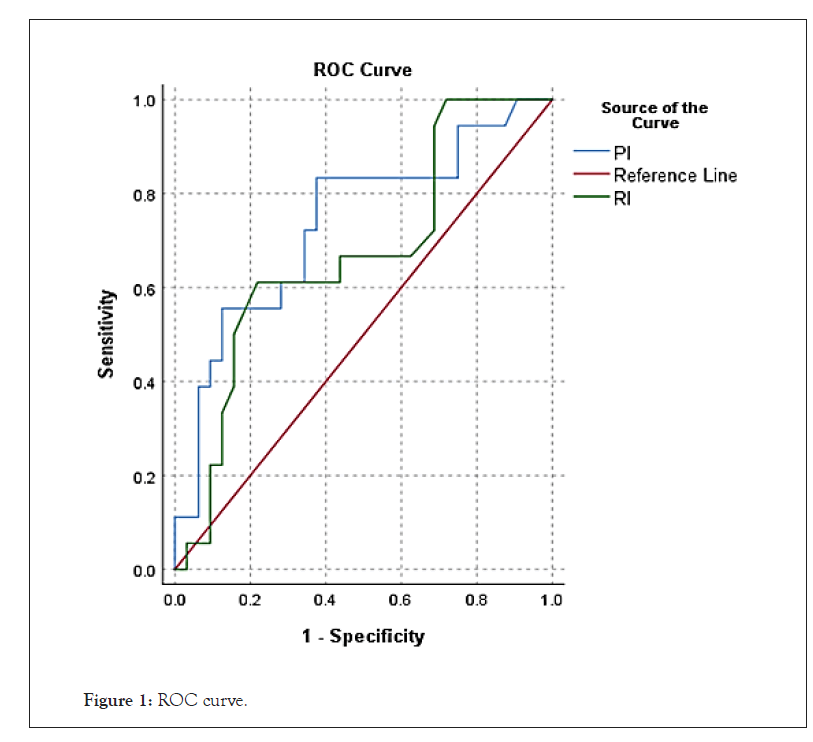
Figure 1: ROC curve.
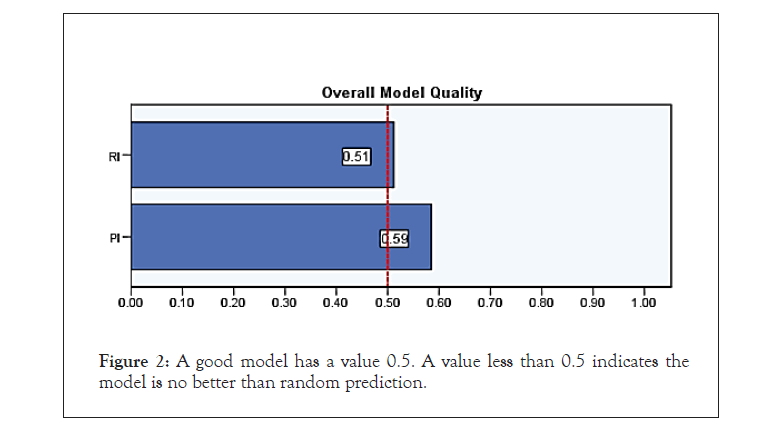
Figure 2: A good model has a value 0.5. A value less than 0.5 indicates the model is no better than random prediction.
An independent sample t-test is performed for testing significance in intratesticular vessel RI, PI with reference to semen abnormalities, abortion and varicocele. It is observed from (Tables 1-3) for semen abnormalities that mean RI and PI shows significant difference i.e. cut-off of RI-0.62 and PI- 1.04 is obtained. Patients having RI and PI values more than cut-off are having more chances of abnormal semen analysis.
| Group statistics | |||||||
|---|---|---|---|---|---|---|---|
| Semen abnormality | N | Mean | Std. deviation | Std. error mean | t-Statistics | p-value | |
| RI | Absent | 32 | 0.5578 | 0.11287 | 0.01995 | -2.26 | 0.028 |
| Present | 18 | 0.6292 | 0.09583 | 0.02259 | |||
| PI | Absent | 32 | 0.8763 | 0.18826 | 0.03328 | -2.687 | 0.01 |
| Present | 18 | 1.0372 | 0.22835 | 0.05382 | |||
Table 1: Group statistics of semen abnormality.
| ROC analysis | |
|---|---|
| Case processing summary | |
| Semen abnormality | Valid N (listwise) |
| Positivea | 18 |
| Negative | 32 |
| Missing | 0 |
| Total | 50 |
Larger values of the test result variable(s) indicate stronger evidence for a positive actual state.
a. The positive actual state is present.
Table 2: Case processing summary.
| Area under the ROC curve | |||||
|---|---|---|---|---|---|
| Test result variable(s) | Area | Std. Errora | Asymptotic Sig.b | Asymptotic 95% confidence interval | |
| Lower bound | Bound upper | ||||
| RI | 0.67 | 0.081 | 0.035 | 0.512 | 0.829 |
| PI | 0.735 | 0.077 | 0.002 | 0.585 | 0.885 |
The test result variable(s): RI, PI has at least one tie between the positive actual state group and the negative actual state group. Statistics may be biased.
a. Under the nonparametric assumption
b. Null hypothesis: true area=0.5
Table 3: Area under the ROC curve.
For other parameters as seen in Tables 4 and 5 for varicocele and abortion, the test is not significant.
| Group statistics | |||||||
|---|---|---|---|---|---|---|---|
| Abortion | N | Mean | Std. deviation | Std. error mean | t-statistics | p-value | |
| RI | Absent | 35 | 0.5694 | 0.10112 | 0.01709 | -1.375 | 0.176 |
| Present | 15 | 0.6163 | 0.13064 | 0.03373 | |||
| PI | Absent | 35 | 0.9326 | 0.21234 | 0.03589 | -0.081 | 0.936 |
| Present | 15 | 0.938 | 0.23151 | 0.05977 | |||
Table 4: Group statistics under the ROC curve.
| Group statistics | |||||||
|---|---|---|---|---|---|---|---|
| Varicocele | N | Mean | Std. deviation | Std. error mean | t-statistics | p-value | |
| RI | Absent | 25 | 0.5896 | 0.10189 | 0.02038 | 0.383 | 0.703 |
| Present | 25 | 0.5774 | 0.12225 | 0.02445 | |||
| PI | Absent | 25 | 0.948 | 0.21234 | 0.04247 | 0.448 | 0.656 |
| Present | 25 | 0.9204 | 0.22287 | 0.04457 | |||
Table 5: Group statistics of Varicocele.
The testes receive its blood supply from internal spermatic arteries through spermatic cord to testes; they form anastomosis with cremasteric and deferential arteries [27]. Testes require adequate blood supply for spermatogenesis, decreasing blood supply may cause ischemic damage. CDUS is one of the most reliable and rapid methods of measuring blood flow, combining anatomical and velocity data, and providing a rapid assessment in routine studies [28,29].
Doppler indices have been used to obtain information about blood flow and vascular impedance that cannot be obtained from velocity information alone .These indices depend on measurement of Peak Systolic Volume( PSV), End Diastolic Volume (EDV) and mean velocity. Two widely used indices are the pulsatility index (PI) and resistive index (RI) [30].
The results of our study showed that the RI and PI of Right testicular artery is significantly different between those who have normal sperm count and those with abnormal sperm count and this indicate its importance as predictive parameter for spermatogenesis. In the study by Ghazy et al. they found that RI could be used as an indicator for spermatogenesis [31]. Study done by lefort et al. concluded that elevated RI is suggestive for ischemia to testes [32].
Studies done on individuals with normal sperm count measuring RI, the results of these studies were Joe et al. found that RI=0.5, in the study by Atilla et al. RI≥0.5, study of Biagiotti et al. RI ≥ 0.5 and Pinggera et al. RI=0.54 , these results nearly similar to our results [30,33-35]. The study done by Pinggera et al. found that (RI >0.6; P<0.001) in oligospermic patients.
There is currently no published explanation for the significant positive relationship between pulsatility index, resistive index and sperm count/ spermatogenesis. Testicular arteries are target organs for androgen and in infertile men testicular arteries have narrow lumen caused by enlarged endothelial cells, a thickened sub-endothelial layer and an abundant adventitia rich in connective tissue fibers and ground substance. The implication is that the anatomical patterns of testicular arteries is related to spermatogenesis ,thus confirming the spectral color doppler traces from the intra-testicular artery can be considered as a marker of spermatogenesis [28]. In another study showed that the mean RI of healthy subjects was 0.54 ± 0.05, and in patients with Abnormal Semen Analysis was 0.68 ± 0.06. This study indicates that the RI of the patients with Abnormal Semen Analysis was significantly higher than the healthy subjects, which was in line with the results of our study. Their results showed that RI with values >0.6 may suggestive of pathological sperm count [36]. Hill- elsohn et al. examined the diagnostic value of RI>0.6 in diagnosing sub-fertile men with spermatogenesis disorder. In their study, the patients were divided into two groups: RI of 0.6 or less (n 1⁄4 49) and RI greater than 0.6 (n 1⁄4 42). The results showed that RI at a threshold of greater than 0.6 had a sensitivity of 63.27% and a likelihood ratio 1.56 to predict overall sperm motility in spermatogenesis. They have concluded that intratesticular RI greater than 0.6 was associated with impaired spermatogenesis. This result supports the idea that evaluating RI using color Doppler sonography can be used as a non-invasive method to evaluate testicular function. Except for one study, other studies stated RI more than 0.6 as a marker of spermatogenesis disorder among men. Like these studies, our results showed a very strong association between RI greater than 0.6 with spermatogenesis disorder in men [18,30,36-40]. Moreover, the RI diagnostic cut point value of more than 0.6 was chosen in these studies, showing that calculating RI using colour doppler sonography is a very powerful modality with acceptable sensitivity and specificity in diagnosis of Abnormal Semen Analysis. In our study we found cut-off for PI also to predict abnormal spermatogenesis which was more significant than RI to be used as a predictor for abnormal spermatogenesis. In our study we also tried to find correlation between spectral doppler parameters and varicocele as well as history of abortion but found no significant correlation.
From the previous data, we can conclude that the use of CDUS as a noninvasive, simple, outpatient procedure for measuring Resistive Index (RI) and Pulsatility Index (PI) parameter of intra- testicular artery branches can be used as a good predictor for spermatogenesis with a cut-off RI (0.62) and PI (1.04).
Citation: Srivastava S, Panchal S, Nagori C, Thaker M (2021) Doppler Parameters of Intratesticular Vessels in Male Factor Sub Fertility. Andrology. 10:230.
Received: 07-Jul-2021 Accepted: 21-Jul-2021 Published: 28-Jul-2021 , DOI: 10.35248/2167-0250.21.10.230
Copyright: © 2021 Srivastava S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.