Short Communication - (2020)Volume 5, Issue 2
Immunocompetent cells were described in the sea star Asterina gibbosa: They recognize specifically various antigens as HRP (Horse-radish peroxydase),Alkaline phosphatase, and trypsin. On the other hand when certain Asterina gibbosa were immunized with Bence-Jones protein, other ones with Rat IGG: A crossed immune reaction occurs. The potential sea star immune system is not broad enough to detect such antigens.
Immunocytochemical; Bence Jones protein; Asterina gibbosa
Recently it was shown that immunized Asterina gibbosa (Asterids Echinodermata) to various enzymes (PeroxydaseAlkalin phosphatase) but also proteins (Trypsin) shown specific immunocytochemical reactions in TEM [1-4].
In the present study we attempt to estimate such reactions by the use of more sophisticated proteins: The Rat immunoglobulin IGG and Light chain of immunoglobulins produced by human myeloma.
These last proteins share common antigenic fragments when injected to various vertebrate hosts.
This The human Bence-Jones protein (Urine) comes from myeloma, a type of cancer: It was used as antigen after purification into Kappa chain of IGG. It is a gift of Saint- Antoine Hospital (Paris France).
Bence Jones protein (BJP) was first described in a patient admitted to St. George’s Hospital in London under the care of Drs. Watson and MacIntyre for vague continuous pain to the chest, back, and pelvis in 1845. Dr. Henry Bence Jones tested this urine and found a substance in it that was precipitated by the addition of nitric acid. Jones proceeded to call this substance “hydrated deutoxide of albumen.” It is important to remember that at that time all urine proteinuria was referred to as albuminuria. After this patient died, his sternum as well as various parts of his vertebrae were noted to be soft and fragile, while multiple hemorrhagic cavities were present in the bone. The patient ’ s cause of death was called “ atrophy from albuminuria.” The actual term, Bence Jones Proteins, was used in 1880 by Dr. Fleischer. Its peculiar characteristics on heating first characterized BJP: Precipitation of the urine at 40°C to 60°C and re-dissolving of the precipitate at 100°C.
While this was a seminal observation that led to the first description of multiple myeloma (MM), it did not hold up to scrutiny over time. Today BJP is known as the light chain of immunoglobulins without the accompanying heavy chain and can be accurately quantified by electrophoretic techniques including immunofixation electrophoresis (IFE). The other crucial teaching point is that BJP is undetectable by dipsticks used to detect proteinuria since they detect albumin and not BJP. In this brief review, we describe the evolution of BJP, its biochemistry, and high clinical relevance [5-7].
In 1939 electrophoresis was applied for the first time to study multiple myeloma by Longsworth. In 1953 Grabar and Williams first described the methods of immunofixation and direct immunoelectrophoresis, which increased detection of small monoclonal light chains not detectable on basic gel electrophoresis, which gave rise to the classic “M spike” seen in the gamma region in patients with multiple myeloma. In 1956, Korngold identified the two classes of BJP: Kappa and Lambda light chains. In 1962, Drs. Edelman and Porter received the Nobel Prize in Medicine for their work in elucidating the chemical structure of antibodies. The M-spike of a particular patient with multiple myeloma was broken down into heavy and light chains. Edelman demonstrated that the light chains of this M-spike were identical to the BJP that the patient excreted in his urine. He expanded on this work by comparing reduced myeloma proteins from different patients. When each of these proteins was reduced, alkylated, and put through starch gel electrophoresis, they exhibited a unique migration pattern. Similar to BJP, when Edelman heated samples containing light chain from normal human serum gamma globulins, they became insoluble and re-solubilized with continued heating. In 1967, Dr. Putnam demonstrated that different BJP had distinct peptide sequences. This differentiation further supported Dr. Edelman’s observation that no two BJP “had the same mobility pattern.”
100 μl [1,2] of a solution at 1 mg/ml of Bence-Jones (BJ) protein were injected to 5 Asterina gibbosa each, twice: 1 injection per week in the coelomic cavity of the animal.
On the other hand, 100 μl of a Rat IGG solution at 1 mg/ml were injected to 5 otherAsterina gibbosa, placed in another aquarium with running sea water.
Other animal controls were injected with HRP (Ref.1) at same manner, at same time. 4 days after the last injection, all axial organs (AO): "the well-known primitive lymphoïd organ" [1,2] were excised and fixed separately in glutaraldehyde (1,5% in cacodylate buffer) then rinsed in the same buffer.
Incubation in antigenic solution (Rat IGG coupled to peroxidase Sigma Products) were performed, except for injected animals to HRP which were incubated in HRP as previously described [3].
A new fixation at glutaraldehyde of 10 minutes occur for all AO (1% in cacodylate buffer). AO were rinced in cacodylate buffer then treated with diaminobenzidin [3] at last dehydrated (Alcohol 70 to Alcohol 100°) and finally embedded in Epon. Cuts were done with a LKB ultrotome. Observations in TEM were realized with a Hitachi Microscope.
Immuuno labelling was seen in treated animals either with Rat IGG, Bence-Jones protein or HRP antigens.
These labelling are situated at the level of perinuclear space (EP) next to Nucleus (N) Reticulum endoplasmic (RE) Golgi apparatus (G) and Lysosomes (L) of the sea star plasmolymphocytes in TEM as previously described (Figure 1).
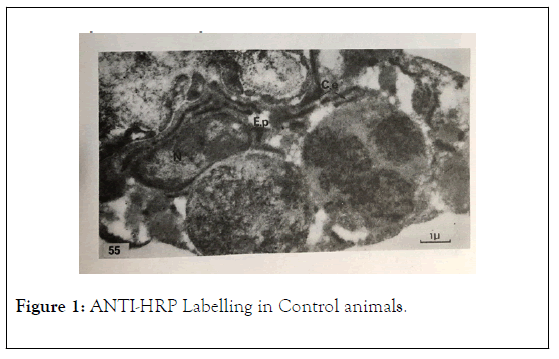
Figure 1: ANTI-HRP Labelling in Control animals.
• Labelling occurs in treated animals to Rat IGG and in a similar way to Bence-Jones protein antigens.
• It is noticeable that sea star Immune system makes no differences between Rat IGG and BJ protein antigens: There is a CROSSED reaction.
• It is obvious that the antigenic structure of Rat IGG and Bence-Jones protein share strong similarities.
• It is the first time, at our knowledge; such incompatibility was described in this invertebrate with the sea star as a model of study.
• Controls anti-HRP and revealed to HRP show a positive immunolabelling.
• We retain that is able to recognize certain antigens but not all the antigens the man does.
The authors declare no conflict of interest.
Citation: Leclerc M (2020) Does the Sea Star Discriminate Bence-Jones Protein from Rat IGG as Antigens? Immunogenet Open Access. 5:128.
Received: 27-Jul-2020 Accepted: 11-Aug-2020 Published: 18-Aug-2020 , DOI: 10.35248/igoa.20.5.128
Copyright: © 2020 Leclerc M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.