
Immunotherapy: Open Access
Open Access
ISSN: 2471-9552

ISSN: 2471-9552
Research Article - (2023)Volume 9, Issue 3
Background: Hepatitis C Virus (HCV) infection causes liver cell injury and ultimately results in liver cirrhosis, fibrosis, and further HCC. Despite current treatment strategies using Direct-Acting Anti-virals (DAAs) to treat HCV patients 40% of HCV infection leads to the development of HCC. The Natural killer cells have a crucial role in an immune response against malignant transformation, including hepatocellular carcinoma. As Natural Killer (NK) cells constitute 25%-50% of liver lymphocytes, they have a significant role to play in liver immunity. A positive correlation exists between the number of Natural Killer (NK) cells in the blood and tumor tissues of HCC patients and their survival and prognosis. The NKG2D receptor has a critical role in the Natural Killer (NK) recognition of target cells. Among different mechanisms, Natural Killer (NK) cell-based innate immune response plays a crucial role in the impairment of adaptive immunity post-HCV infection. Natural Killer (NK) cells kill the virus-infected cells through cytolysis, which requires direct contact of Natural Killer (NK) cells with the target cell, and immunological synapse formation. NKG2D is an important Natural Killer (NK) cell receptor, which is activated upon HCV infection resulting in increased cytotoxicity of Natural Killer (NK) cells. Thus, Natural Killer (NK) cells distinguish between normal cells and virus-infected cells through the interaction of NKG2D with its ligands on virus- infected cells. Therefore, Natural Killer (NK) cells are known to contribute to the control of HCV infection itself and have anti-cancer effector functions. Natural Killer (NK) cells being an integral part of the host immune defense against HCV-associated HCC, studying related molecular cascades will provide a newer direction for evolving better predictive biomarkers and designing effective immunotherapeutic strategies.
Methods: A total of 25 HCV infected patients, Chronic HCV (n=15), HCV and HCV related HCC (n=10) and 5 non-infected age and gender matched control subjects were taken in study. Both pre and post-DAA treated patients were included in this study.
Results: The present study has been undertaken to investigate the differential expression analysis of Natural Killer (NK) cell receptors in hepatitis C virus-infected with chronic hepatitis risk of developing HCC.
Conclusion: NKG2D receptor expression and Natural Killer (NK) cell percentage in peripheral blood could provide potential biomarkers for HCC detection and progression.
Hepatocellular carcinoma; Hepatitis C virus; Natural killer cells-NKG2D receptor
HCV: Hepatitis C Virus; DAAs: Direct-Acting Anti-virals; NK: Natural Killer; HCC: Hepatocellular Cancer; NKG2D: NK Group 2D; NKG2DLs: NKG2D Ligands; MHC: Major Histocompatibility Complex; PI3K: Phosphatidylinositol 3-hydroxykinase; PLCG2: Phospholipase C Gamma 2; JNK: c-Jun-NH(2)-terminal Kinase; ADCC: Antibody-Dependent Cellular Cytotoxicity; KIR: Killer Immunoglobulin-like Receptor; Th1: T-Helper cells type 1; IRB: Institutional Review Board; ELISA: Enzyme-Linked Immunosorbent Assay
Hepatitis C Virus (HCV) infection is a serious health concern and the leading cause of liver disease worldwide. Although present medications are helpful, they are costly and have unfavorable side effects. Understanding how HCV interacts with host cells could lead to the discovery of new cellular targets for developing innovative treatments. Liver cirrhosis and Hepatocellular Cancer (HCC) develop in approximately 20% of patients with persistent viremia. During acute HCV infection, innate and adaptive immune systems work together to resolve the infection. In contrast, HCV has sophisticated systems that allow it to evade both host defenses. As a result, the immune response in chronic HCV infection changes over time accompanied by disease progression.
Natural Killer (NK) cells are the main type of innate immune cells within the body that play a crucial role in the fight against the human Hepatitis C Virus (HCV) infected cells and malignant cells [1].
One of the main activating receptors needed for recognizing target cells is the NK Group 2D (NKG2D) receptor. NKG2D Ligands (NKG2DLs) are remotely related to Major Histocompatibility Complex (MHC) class I molecules. They bind to the NKG2D receptor in response to cellular stress, viral infection, or malignant transformation, marking stressed cells for removal by NKG2D+ lymphocytes. Hence, the NKG2D receptor acts as a sensor that decodes cellular stress into activation signals for immune cells.
Hepatitis C Virus (HCV) is a hepatotropic flavivirus and a major cause of chronic viral hepatitis, liver cirrhosis, and Hepatocellular Carcinoma (HCC) [2]. Approximately 55-85% of cases presenting with acute HCV infection progress to chronicity, with 20-30% developing liver cirrhosis, and 1-4% progressing to hepatocellular carcinoma [3]. Due to undiagnosed disease and lack of treatment availability, an estimated 1-3% of the world population currently lives with HCV infection [4]. The prevalence of HCV infection is particularly high in low-income countries within Asia (0.4-6.8%), North Africa/Middle East (2.5-3.9%), and sub-Saharan Africa (1.0- 5.3%) [5,6]. Lower incidence is seen in the Oceania (0.1-1.5%), the Caribbean (0.2-1.3%), Europe (0.9-3.3%), and the Americas (0.9-1.9%) [5,6]. Despite these established ranges, countries such as Cameroon (13.8%), Burundi (11.3%), and Gabon (9.2%) have particularly high HCV seroprevalence due to limited availability of HCV screening and treatment strategies [5,6].
The antiviral immune response to hepatitis C is traditionally understood to be driven by the adaptive immune cells such as B cells and T cells, and more recently by innate immune cells, specifically Natural Killer (NK) cells. While adaptive immunity and hence antigenic specificity of B and T cells to viral infection is generated through somatic rearrangement of antigenic receptors, it is significantly slower than the innate immune response. NK cells possess an innate ability to provide a fast acting and potent response to cells deemed harmful, such as cancer and virus-infected cells, which is controlled by a number of NK cell receptors.
The activating receptor NKG2D is overexpressed in patients with Hepatocellular Carcinoma (HCC) (Hwang, et al.,) [7]. In response to NKG2D binding, several signaling pathways may be activated, including Phosphatidylinositol 3-hydroxykinase (PI3K), Phospholipase C Gamma 2 (PLCG2), and c-Jun-NH(2)-terminal Kinase (JNK) [8]. As a result, NK cells produce more antitumor effects by enhancing Antibody-Dependent Cellular Cytotoxicity (ADCC), secreting cytokines, and initiating apoptosis. As a result, NKG2D receptors may facilitate HCC progression, which might affect immunotherapy for HCC.
In humans, NK cells are identified based on their expression of activating Fc receptor, CD16 (FcγRIIIA), CD56 and lack of CD3. Recent studies have classified the various stages of human NK cell development into six phases, of which the final three represent mature NK cells [9]. Phase 4 marks the transition of immature NKs into mature NK cells expressing NKp80, NKG2D, CD335, CD337, and CD161 [10]. Phase 5 represents circulating NK cells, and is characterized by an observable spike in CD56 expression representing the relatively young CD56 bright population, as well as the more mature CD56 dim population that co-express CD16 and Killer Immunoglobulin-like Receptor (KIR) (CD158). Phase 6 represents terminal maturation gaining expression of CD57 and may include the generation of “adaptive” or “memory-like” NK cells following antigen exposure, gaining expression of NKG2D and KLRG1 [11,12].
Almost 10% of NK cells in the peripheral blood and roughly 100% of NK cells in secondary lymphoid tissues have a high surface expression of CD56 (CD56 bright) and are CD16 low, acting as potent immunoregulatory cells via the secretion of cytokines such as IFN-γ which contribute to the priming of T-Helper cells type 1 (Th1) [13,14].
Moreover, in response to HCV infection, NK cell concentration in the liver increases. The infiltration of NK cells into the liver is mediated by chemokines such as CCL2, CXCL2, CXCL9, CXCL10, and a plethora of cytokines such as the TGF-β, IL-12, and IL-18, secreted by hepatocytes, Kupffer cells, liver sinusoidal endothelial cells, and T cells [15,16]. As significant antiviral effectors in the liver, both resident and liver-infiltrating NK cells are a major component of the immune response against HCV infection, as outlined below. Nonetheless, the vast majority of studies examine circulating NK cells due to ease of sample collection, and therefore NK cells examined in this review will represent blood NK cells unless otherwise stated.
Study design
A total of 25 HCV infected patients, Chronic HCV (n=15), HCV and HCV related HCC (n=10) and 5 non-infected age and gender matched control subjects were taken in study. Both pre and post- DAA treated patients were included in this study. Informed consent in writing was obtained from each individual after taking approval from the Institutional Review Board (IRB), Centre for Liver Research and Diagnostics, Deccan College of Medical Sciences, Hyderabad.
Inclusion criteria
• Patients clinically diagnosed as chronic hepatitis C
• Age: ≥ 18 years
• Gender: Both males and females
• Viral load ≥ 1,00,000 copies/ml
Exclusion criteria
• Pregnant women
• Decompensated liver patients
• Alcohol liver-diseased patients
• Multiple sex workers
• Patients on any corticosteroids or laser therapy
• Injection drug users
• Any co-infected samples with other viruses like HIV and HBV were excluded from the study
Collection and preparation of human sera from healthy and HCV-infected individuals
A total of 25 peripheral blood samples (5 from healthy and 15 HCV infected individuals and 10 HCV-related HCC) were collected after taking informed consent forms from all the subjects visiting the outpatient ward at Centre for Liver and Diagnostics- DCMS Further HCV QPCR analysis HCV diagnosis using antibody testing and quantitative PCR and sequencing analysis for 5’UTR confirmed the serum antibody as well as HCV RNA with appropriate genotype in infected individuals whereas sera obtained from subjects without any such history of viral infection were considered as healthy controls.
Detection of HCV antibodies
ORTHO HCV, Enzyme-Linked Immunosorbent Assay (ELISA) for the detection of antibodies to Hepatitis C Virus (anti-HCV) in human serum or plasma. Approximately 30 minutes prior to the procedure, the kit components were placed at room temperature (20 to 30°C) and the reagents are inverted gently several times (without foaming). The incubator temperature was maintained at 37°C ± 1°C.
HCV viral load estimation
Viral RNA was extracted from all samples using High Pure Viral RNA Kit (Roche) and quantified HCV viral load by amplifying 5'UTR using Real-time primers and Taqman probes.
Liver Function Test (LFT)
Biochemical parameters specific to LFT were estimated in serum samples by using their respective kits as per the manufacturer’s instructions. The LFT values obtained from the patient samples will be compared with the healthy controls.
Isolation and storage of Peripheral Blood Mononuclear Cells (PBMCs)
PBMCs were isolated by use of density-gradient separation using Ficoll Hypaque (Pharmacia) and stored in liquid nitrogen for further analysis purposes.
Immunostaining and flowcytometry of NK cells
All phenotypic and functional NK cell analyses were performed on frozen and subsequently thawed PBMCs. PBMCs will be incubated with the following Abs: CD56-FITC (BD Biosciences-Pharmingen, San Jose, CA); NKG2D-PE (R&D Systems, Inc., Minneapolis, MN) and will be analyzed on a BD Biosciences flow cytometer (FACSCalibur or FACSCanto).
NK cell expression analysis
Total RNA was isolated from PBMCs, cDNA was constructed using reverse transcriptase enzyme, and expression analysis will be done by SYBR-Green based quantitative RT-PCR in all selected individuals. cDNA from all the variables were quantified by nanodrop reading and 5 ng of cDNA from each sample will be used for quantitative PCR. The reaction mixture was prepared for all the primers as follows: 2X SYBR Green Mix 10 μl, NKG2D, Forward primer (10 pmol/μl), 0.5 μl, NKG2D, Reverse primer (10 pmol/μl) 0.5 μl, cDNA template 2 μl and dH2O 7 μl. Reaction condition will be adjusted as per the melting temperature (Tm) of each primer following first step of initial denaturation at 94°C for 5 min, the second step for 40 cycles of denaturation at 94°C for 30 sec, annealing at 54°C for 40 sec and extension at 72°C for 40 sec. Final denaturation will be kept at 72°C for 5 min followed by melting curve analysis for 15 min. Melting curve analysis will be performed to separate the amplified products from primer dimers. The primer specific to GAPDH will be used as an endogenous control for the normalization of the test samples. Ct values for all the samples will be noted and used for statistical analysis for fold differences. Each reaction will be performed in triplicate to avoid any technical error.
Detection of HCV antibodies
ORTHO HCV, Enzyme-Linked Immunosorbent Assay (ELISA) for the detection of antibodies to Hepatitis C Virus (anti-HCV) in human serum or plasma. Approximately 30 minutes prior to the procedure, the kit components were placed at room temperature (20 to 30°C) and the reagents are inverted gently several times (without foaming). The incubator temperature was maintained at 37°C ± 1°C. All the patients were found to be Anti-HCV Positive.
Analysis of HCV viral load
Quantitative PCR and sequencing analysis for 5’UTR confirmed the load of HCV RNA in chronic Hepatitis patients (N=15), Hepatocellular Carcinoma (10) infected individuals whereas sera obtained from subjects without any such history of viral infection were showed as healthy controls.
All patients recruited in the study were found to be HCV antibody positive and had a viral load of more than 1,00,000 copies per ml (Table 1). HCV diagnosis using antibody testing and quantitative Demographic details such as age and gender along with high viral load (>1,00,000 copies/ml) were collected from every subject enrolled in the study.
| S. No | Age/Gender | Viral load (copies/ml) |
|---|---|---|
| 1 | 32/M | 3,48,150 |
| 2 | 55/M | 8,48,780 |
| 3 | 49/M | 3,18,246 |
| 4 | 35/M | 17,44,668 |
| 5 | 60/M | 1,18,260 |
| 6 | 43/M | 3,30,450 |
| 7 | 73/M | 40,25,780 |
| 8 | 35/M | 43,96,750 |
| 9 | 58/F | 8,12,560 |
| 10 | 23/M | 25,10,840 |
| 11 | 57/M | 28,45,260 |
| 12 | 45/F | 1,29,541 |
| 13 | 50/M | 15,08,170 |
| 14 | 60/M | 60,15,000 |
| 15 | 45/M | 12,84,610 |
Table 1: Demographic details of HCV-infected subjects enrolled in the study.
NK cells are usually abundant and play an important role in early viral clearance in HCV-infected patients; however, the numbers of NK cells are lower in both peripheral blood and the liver in chronically infected HCV patients. Biochemical liver function parameters were assessed for HCV-infected individuals and confirmed for suitability of utilizing to conduct the study (Table 2).
| Biochemical parameters | HCV infected (Mean ± SD) |
|---|---|
| ALT | 65.42 ± 12.12 |
| AST | 58.35 ± 11.74 |
| ALP | 396.17 ± 43.56 |
Note: ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; ALP: Alkaline Phosphatase.
Table 2: Important biochemical parameters were reported for HCV- infected patients enrolled in the study.
Immunostaining and flowcytometry of NK cells
All phenotypic and functional NK cell analyses was performed on frozen and subsequently thawed PBMCs. PBMCs will be incubated with the following Abs: CD56-FITC (BD Biosciences-Pharmingen, San Jose, CA); NKG2D-PE (R&D Systems, Inc., Minneapolis, MN) and will be analyzed on a BD Biosciences flow cytometer (FACSCalibur or FACSCanto).
The flow cytometric analysis data showed a decrease in NK cells in peripheral blood collected from HCV Positive patients.
NKG2D q-PCR expression analysis
Total RNA was isolated from PBMCs, cDNA was constructed using Reverse transcriptase enzyme, and expression analysis will be done by SYBR-Green based quantitative RT-PCR in all selected individuals. cDNA from all the variables were quantified by nanodrop reading and 5 ng of cDNA from each sample will be used for quantitative PCR (Figure 1).
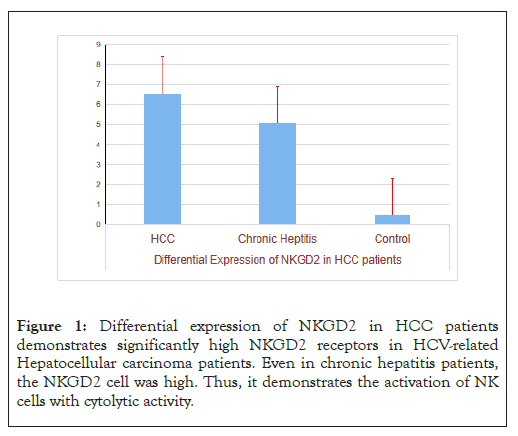
Figure 1: Differential expression of NKGD2 in HCC patients demonstrates significantly high NKGD2 receptors in HCV-related Hepatocellular carcinoma patients. Even in chronic hepatitis patients, the NKGD2 cell was high. Thus, it demonstrates the activation of NK cells with cytolytic activity.
HCV infection is a key contributor to extensive immune infiltration HCC development and progression leads to HCC development. HCC is one of the leading causes of cancer-related deaths worldwide with limited treatment options. This has opened the need for channels for developing targeted immunotherapy. Hence, understanding the activation of NKG2D+NK cells contributes to HCV progression to liver cirrhosis and HC. NKs act as an important cell component in innate immunity, which is essential for attacking and neutralizing microorganisms and eliminating aberrantly transformed cells [17].
In HCV infection, NK cells have been implicated in nonspecific immune clearance, as well as the initiation and modulation of specific antiviral immune responses. Studies by Wang, et al., NKG2D controls the worsening of liver inflammation by activating NK cells. According to this finding supports the idea that NKG2D has a protective effect that extends beyond NK cell-mediated cytotoxicity and cytokine generation. According to Jelenčić, et al., NKG2D is found on the earliest progenitors of the NK cell lineage and plays an important role in NK cell development by causing changes in NK cell subsets and inducing faster division of immature NK cells in the bone marrow [18]. Moreover, NKG2D stimulates the immune system by promoting the development of innate-like B cells.
This study revealed that NKG2D gene expression increased significantly in the HCC group compared with those in the CHCV and liver cirrhosis groups. Meanwhile, NKG2D+NK% showed no significant difference between the CHCV and liver cirrhosis groups. Liu, et al., have reported that NK cells are involved in the first line of immune defense against viral infections and tumors [19].
Wang, et al., have specifically highlighted the role of NKG2D+NK cells in tumor transformation and HCC [9]. NKG2D can be widely expressed in hepatoma cells. Olivero, et al., and Bonorino, et al., described the frequency of NK cells appears to decrease concomitantly with disease progression [20,21].
The various strategies employed by HCV to evade NKG2D pathway immune responses are promising immunotherapy targets. A combination of NK cell-based immunotherapy with chemotherapy or other multiple therapies has the potential to be more effective for HCC patients and patients with persistent HBV/HCV infections. Thus, the Potential biomarkers for the progression and identification of HCC could be obtained by measuring NK levels in peripheral blood and looking for NKG2D receptor expression in NK cells.
Authors have no competing interests to declare.
Not Applicable.
The data used to support the findings of this study are available in this article.
We sincerely thank all reviewers for their constructive comments and informative suggestions.
Authors have no conflict of interest to declare.
RR and AAK contributed equally to this work; RR, VAA, and AAK designed the study; GMA, VAA, AAK conducted the experiments; AAK, VAA wrote the manuscript. All authors reviewed and approved the final manuscript.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Rozati R, Ayapati VA, Ayapati GM, Khan AA (2023) Differential Expression Analysis of Natural Killer (NK) Cell Receptor (NKG2D) in Hepatitis C Virus-Infected Chronic Hepatitis and Hepatocellular Patients for the Development of Targeted Immunotherapy. Immunotherapy (Los Angel). 9:235.
Received: 17-Aug-2023, Manuscript No. IMT-23-26159; Editor assigned: 21-Aug-2023, Pre QC No. IMT-23-26159 (PQ); Reviewed: 04-Sep-2023, QC No. IMT-23-26159; Revised: 11-Sep-2023, Manuscript No. IMT-23-26159 (R); Published: 18-Sep-2023 , DOI: 10.35248/2471-9552.23.09.235
Copyright: © 2023 Rozati R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.