Journal of Nanomedicine & Biotherapeutic Discovery
Open Access
ISSN: 2155-983X
ISSN: 2155-983X
Research Article - (2018) Volume 8, Issue 1
Keywords: Bio-flexy films; Nanosized Topiramate; Soft palatal delivery, Solanum melongena biopolymer
Sodium CMC: Sodium Carboxyl Methyl Cellulose;
FMO: Bio-Flexy Film Formulation of Topiramate with Solanum melongena biopolymer;
FSO: Bio-Flexy Film Formulation of Topiramate with Sodium CMC standard polymer;
GIT: Gastro Intestinal Tract;
U.V: Ultraviolet Visible Spectroscopy;
I.R: Infra-Red Spectroscopy;
SEM: Scanning Electron Microscopy;
DSC: Differential Scanning Calorimetry;
NMR: Nuclear Magnetic Resonance;
cm2: Centimeters Square;
mins: Minutes;
mm: Millimeters;
No.: Number;
mL: Milliliters;
g: Grams;
mg: Milligram;
rpm: Revolutions per minute;
KBr: Potassium Bromide
The soft palate (muscular palate) is the soft tissue constituting the back of the roof of the mouth. It does not contain bone, is non-keratinized, leads to systemic absorption of drug [1]. It possesses surface area of about 200 cm2 and thickness of 158-224 μm. Targeting of drugs to brain is difficult to achieve due to Blood Brain Barrier. Oro-Trans-Soft Palatal delivery provides a novel, unique platform for drug delivery for targeting to brain. It is for retentive drug delivery and is acceptable to patients. Nanosized drug loaded mucoadhesive bio-flexy films were designed so as to provide significant permeability and sustained drug action. It is suitable for and protein, potent peptide delivery. Soft palate is innervate by Accessory Meningeal artery; Middle Meningeal artery; Greater Palatine branch of maxillary artery; ascending pharyngeal artery, ascending palatine branch of facial artery [2]. It has enriched nerve supply Lesser palatine nerve; greater palatine nerve; nasopalatine nerve; glossopharyngeal nerve; motor nerves of mandibular branch of trigeminal nerve (Cranial nerve V). Nanosized drug can directly reach to brain through trigeminal nerve via inter and intra neural pathway [3].
As per survey report, presently Epilepsy ranks 7th position causing 3.3% total deaths worldwide, that is expected to reach to 6th position causing 3.7% of total deaths by year 2030. Topiramate is anticonvulsant drug available in tablets form undergoes pre-systemic metabolism in liver resulting in retardation of drug action. In this study, a non-reactive, bio-safe, economic biopolymer was isolated from pulp of Solanum melongena. It was devoid of toxicity, had inbuilt-biodegradability being obtained from natural edible source. Solanum melongena contains vitamins, minerals, dietary fiber, proteins, antioxidants, glucoside, phenolic compounds (caffeic, chlorogenic), flavonoids (nasunin, delphindin), carbohydrates, vitamin A, linoleic, omega 3 and omega 6 fatty acids, folate, calcium, magnesium, phosphorus, potassium, glutamate and proline [4]. Soft palate being non-viable, non-invasive, offers high mucoretentivity, bioavailability, minimal doses, bypasses first-pass metabolism by gastrointestinal tract [5]. Topiramate, anticonvulsant drug possesses t1/2:19-30 hours; protein binding: 13-17%; water solubility: 9.8 mg/L. It reduces glutamatergic neurotransmission. Adverse effects are abdominal pain, pharyngitis, suicidal thoughts and sudden unexpected death. In order to reduce frequency of drug dose and to minimize side effects, Topiramate in nanosized form was developed as bio-flexy films formulations so as to achieve prolonged drug action for 48 hours.
Importance and advantages of using biopolymer instead of using synthetic polymers like carboxyl methyl cellulose and hydroxyl propyl methyl cellulose [5]
1. Isolated biopolymer exhibited significant biodegradability, mucoadhesivity, filmability, retardability and comparable to synthetic polymers.
2. Economically cheap and environment friendly.
3. Suitable as a drug carrier for sustained release dosage forms with suitable modification.
4. It can applicable in Pharmaceutical Industries and commercialized effectively.
5. Solanum melongena biopolymer is having uniqueness of being pure, natural origin isolated Green brinjal pulp.
6. Solanum melongena biopolymer is isolated using acetone as solvent that belongs to Class 3 so is less toxic, possess lower risk to human health than Class 1, 2 carcinogenic solvents used.
7. Hydroxyl Propyl Methyl Cellulose, Carboxyl Methyl Cellulose are synthesized using various harmful chemicals. Thus, biopolymer avoids toxic effects of synthetic polymers.
8. Biopolymer serves as a suitable drug carrier for the formulation of bio-flexy films.
Drug-Topiramate (obtained from Sun Pharmaceuticals Industries Limited., Dahej, Gujarat)
Polymers-Solanum melongena biopolymer (Green brinjals obtained from local market)
Sodium CMC (Sodium Carboxyl Methyl Cellulose) (Central Drug House (P) Limited. New Delhi)
Analytical grade reagents were used. The experimental work was carried out by using double distilled water.
Isolation of biomaterial from Solanum melongena
Weighed 250 g of Solanum melongena, removed skin. Prepared slurry using 500mL of distilled water filtered using muslin cloth. Propan- 2-one was added in optimized quantity in ratio of 1:2 to the filtrate. Refrigeration done for 24 hours. Centrifugation was done for 15 min at 3500 rpm. The residue was collected and supernatant was discarded. Kept biomaterial for natural drying for 24 hours. The obtained biopolymer was then powdered, sieved through Sieve Number 120. It was packed and stored for further use. Optimized six times, reported % yield.
Physical and chemical characterization of the isolated biopolymer
The isolated bio-material was characterized for its physicochemical properties [5].
a. The physical parameters included colour, odour, melting point, solubility [5].
b. Chemical tests included test for presence of carbohydrates, proteins and starch.
I. Molisch reagent test for presence of Carbohydrates:
• To 2 mL of aqueous solution of biopolymer in test tube, added 2 drops of Molisch reagent.
• Poured the solution slowly into another test tube that is containing 2 mL of concentrated Sulphuric acid. Formation of 2 layers and appearance of purple colour at interface occurred. It was due to the formation of 5-hydroxy methyl furfural.
II. Biuret test for presence of Proteins:
• To 2 mL of aqueous solution of biopolymer in test tube, 1 mL of 1% Sodium Hydroxide solution was added.1% Copper (II) Sulphate solution was then incorporated drop wise followed by vigorous shaking. The mixture was allowed to stand for 5 minutes. Colour change was observed. Cu (II) ions form a violet-coloured chelate complex that absorb light at 540 nm. This indicated presence of proteins.
III. Test for presence of Starch:
• To 2 mL of aqueous solution of biopolymer in test tube, added 1-2 drops of Iodine solution and the colour change was observed. An intense blue black colour appeared. This was due to formation of starch-iodide complex that causes charge transfer among starch and iodide ions as it changes the spacing between the energy orbitals.
IV. Test for presence of Reducing Sugar:
• To 2 mL of aqueous solution of biopolymer in test tube, 1 mL each of Fehling’s A and B solutions were added. Heated the test tube at 60°C until a brick red precipitate appeared indicating the presence of reducing sugar presence. It was due to formation of insoluble Copper Oxide.
Drug-excipient interaction study [5]
In this study 3 different ratios of Topiramate: isolated Solanum melongena biopolymer i.e., 1:1, 1:3 and 3:1 were taken. Absorbance was measured and compared with that of pure Topiramate.
a) Dry method: Drug-biopolymer were taken in ratios of 1:1, 1:3 and 3:1 were taken in three petridishes in dry form, kept at room temperature for about two hours. The mixtures were then diluted by using 2 mL methanol. Measured absorbance and reported λmax shift in comparison with that of pure Topiramate.
b) Wet method: Drug-biopolymer were taken in ratios of 1:1, 1:3 and 3:1 were taken in three petridishes. The mixtures were wetted with 1 mL of distilled water followed by drying at 50°C for 30 min in oven. The mixtures were then diluted by using 2 mL methanol. Absorbance was measured and λmax shift was reported by comparing with that of pure Topiramate.
Importance of determination of drug-biopolymer interaction by dry and wet methods
The two methods revealed that no interaction of drug-biopolymer occurred either in dry form on in presence of solvent. Since biopolymer is isolated from natural source and used in formulation, it has to be ascertained whether the biopolymer is inert in both dry (during storage) as well as wet (if used in oral drug delivery) conditions. Thus to confirm inertness and non-reactiveness of biopolymer with drug, these two methods had been performed. The drug was found to be intact with biopolymer.
Spectral analysis of isolated Solanum melongena biopolymer [5,6]
FTIR Spectra: The IR spectroscopy of isolated biopolymer in solid form was performed by using Potassium Bromide Disc Method. 1mg of sample was finely admixed with about 100 mg of Potassium Bromide (KBr) in mortar. Pressure of 10 tons was applied to mixture using hydraulic pump. Small pellet of 1-2mm in diameter was formed. The prepared pellet was kept in path of IR radiation and recorded the spectrum within the range of 4000-200cm-1.
DSC (Differential scanning calorimetry): Amount of the heat difference of sample and reference was measured against temperature. It was performed for determination of Glass Transition temperature (GTT or Tg). For DSC the Perkin Elmer Instrument, Model-JADE DSC will be used, with the Heat flow of 50-250°C at the rate of 10°C/minute and Nitrogen rate of flow of 20 ml/minute was used.
NMR (Nuclear magnetic resonance) spectral analysis: Exploits the magnetic properties of atomic nuclei, determines the physical and chemical properties of atoms or the molecules in which they are contained. It relies on the phenomenon of nuclear magnetic resonance and can provide detailed information about the structure, dynamics, reaction state, and chemical environment of molecule. Solvent used was DMSO (Dimethyl Sulfoxide). The spectrometer was connected to flow cell of 5 mm diameter. High flow rates were applied to the sample, a valve switch was activated to stop the flow for quick measurement. When the valve switches back, the flow cell in the instrument was rinsed again with the reaction mixture. The spectrum was sent to the automation computer where it can be processed and analyzed.
Topiramate standard curve preparation [7,8]: 100 μg/mL Stock solution of Topiramate was prepared by dissolving 10 mg of Topiramate in 30 mL of Buffer (pH 7.4) in a 100 mL volumetric flask and made up the volume up to 100 mL with Buffer (pH 7.4). From the prepared stock solution, dilutions of 0.5,1, 2, 3, 4 and 5 μg/mL Concentrations were prepared in 10 mL volumetric flasks and made up volume up to 10 mL with Buffer (pH 7.4) Measured absorbance at 444 nm using with Buffer (pH 7.4) as blank.
Solvent evaporation method: 100 mg Topiramate was admixed with 5 mg of fructose, 10 mg of dextrose and 10 mL of Methanol in mortar pestle. Sonication of mixture was performed for 5 cycles (180 sec/cycle) in ultrasonic bath sonicator. The mixture was then diluted with 50 mL distilled water and sonicated up to 15 cycles. Measured % Transmittance, Absorbance, % Blockage (100 – % Transmittance) after every 5 cycles. The residue was then dried and stored for further use.
Sonication method: 100 mg Topiramate was admixed with 5 mg of fructose, 10 mg of dextrose and 10 mL of Distilled water in mortar pestle. Sonication of mixture was performed for 5 cycles (180 seconds/ cycle) in ultrasonic bath sonicator. The mixture was then diluted with 50 mL distilled water and sonicated up to 15 cycles. Measured % Transmittance, Absorbance, % Blockage (100% Transmittance) after every 5 cycles. The residue was then dried and stored for further use.
The main purpose of Nanosizing Topiramate by two different methods was to compare novel sonication method with published standard solvent evaporation method (Figure 1).
Formulation of bio-flexy films (Solvent casting method)
Nanosized Tiagabine and Solanum melongena biopolymer solution (in ratios of 1:0.5, 1:1, 1:3, 1:5, 1:6, 1:10) were taken. Optimized quantities of flexicizers (Fructose, Dextrose), Plasticizer (Glycerine), film initiator (Pectin) were incorporated. Added 20 mL of solvent (Distilled water). Mixture was triturated uniformly for 2 mins. Magnetic stirring was performed for 15 mins. Sonication was done up to 5 cycles (each cycle 180 seconds). Nanodispersions were transferred in to petridishes and naturally dried for 24 hours at room temperature. 0.1 mL of 1% borax solution (hardening agent) was used for removing of prepared films from petridishes. Checked filmability of prepared films. Same procedure was followed for formulations of standard polymer films (Tables 1 and 2).
| Formulation | FMO1 | FMO2 | FMO3 | FMO4 | FMO5 | Role of ingredients |
|---|---|---|---|---|---|---|
| Drug: biopolymer ratios | (1:1) | (1:3) | (1:5) | (1:6) | (1:10) | |
| Topiramate (mg) | 100 | 100 | 100 | 100 | 100 | Anticonvulsant |
| Solanum melongena biopolymer (mg) | 100 | 300 | 500 | 600 | 1000 | Retardant, mucoadhesive agent, film former |
| Fructose (mg) | 50 | 50 | 50 | 50 | 50 | Flexicizer |
| Dextrose (mg) | 100 | 100 | 100 | 100 | 100 | Flexicizer |
| Glycerine (µl) | 10 | 10 | 10 | 10 | 10 | Plasticizer |
| Pectin (g) | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | Film initiator |
| Distilled water (mL) | 20 | 20 | 20 | 20 | 20 | Solvent |
Table 1: Formulation of Nanosized Topiramate loaded bio-flexy films using Solanum melongena biopolymer.
| Formulation | FSO1 | FSO2 | FSO3 | FSO4 | FSO5 | Role of ingredients |
|---|---|---|---|---|---|---|
| Drug: Polymer ratios | (1:1) | (1:3) | (1:5) | (1:6) | (1:10) | |
| Topiramate (mg) | 100 | 100 | 100 | 100 | 100 | Anticonvulsant |
| Sodium carboxyl methyl cellulose standard polymer (mg) | 100 | 300 | 500 | 600 | 1000 | Film former |
| Fructose (mg) | 50 | 50 | 50 | 50 | 50 | Flexicizer |
| Dextrose (mg) | 100 | 100 | 100 | 100 | 100 | Flexicizer |
| Glycerine (µl) | 10 | 10 | 10 | 10 | 10 | Plasticizer |
| Pectin (g) | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | Film initiator |
| Distilled water (mL) | 20 | 20 | 20 | 20 | 20 | Solvent |
Table 2: Formulation of Nanosized Topiramate loaded flexy films using (Sodium carboxyl methyl cellulose) (Sodium CMC) standard polymer.
Evaluation parameters for nanosized topiramate loaded films Mucoretention time (Dynamic method: Rotating cylinder method):
In this method, the mucoadhesivity of formulated films was evaluated on intestinal mucosa of Capra aegagrus (i.e., goat). Bio-flexy films of area 1cm2 of each formulation were cut down using sharp blade. Tied the goat intestinal mucosa over the rotating basket of i-disso apparatus. The Dissolution media was 900 mL of buffer (pH 7.4), maintained at 37°C, subjected for rotation at 50 rpm. It was applied over the inner surface of goat intestinal mucosa until it got dislodged. The dislodgement and detachment of films from mucosal surface was observed at regular intervals.
Mucoadhesion study by static method: Bio-flexy films of area 1cm2 of each formulation were cut down using sharp blade. Tied the Capra aegagrus (goat) intestinal mucosa over slanting condenser over which buffer was allowed to flow from a burette. It was applied over the inner surface of goat intestinal mucosa until it got dislodged. The detachment and dislodgement of film from mucosal substrate was noted at regular intervals.
Swelling percentage study: It was determined as increase in weight and area because of Swelling. 1 × 1 cm2 sized films were weighed, transferred in petridish and added 10 mL of distilled water. After one hour, reweighed the films. Absorption of water and swelling of films caused increased in weights of films. The study was performed for 24 hours.
Thickness of films: Standard Digital Micrometer was used to determine thickness of formulated films.
Tensile strength of films: Tensile strength of films determined by using a rectangular frame device having 2 plates, one of which is movable while other plate is stationary. Movable plate could be pulled by loading weights on the attached string. Each film was fixed between the stationary and movable plate. The Tensile strength was calculated by measuring the total weight that was loaded on the string that caused breakage of film.
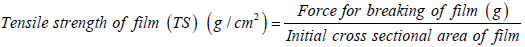
Folding endurance of films: Calculated manually by repeated folding 1 film at same place until it broke or up to 300 times.
Surface pH: Surface pH of formulated films was determined by using digital pH meter. It should be neutral or close to soft palatal pH otherwise formulation might cause irritation to soft palatal mucosa. The formulated bio-flexy films were kept in contact with 1 mL of distilled water at room temperature for 1 hour. The pH was measured in triplicate. Compatibility of formulations with soft palatal pH is essential.
Weight uniformity: Weight uniformity of formulated films was determined by weighing 10 formulations of 1 cm2 diameter and determined average weight.
Drug content uniformity: Drug Content uniformity of formulated films was calculated by dissolving the films in phosphate buffer (pH 7.4) (100 ml) for 24 hours with occasional shaking. Diluted 5 mL of solution with phosphate buffer pH 7.4 up to 20 ml. Filtered through Whattman filter paper of 0.45 mm. The drug content determined by UV analysis at λmax 750 nm.
Percentage moisture uptake (PMU): PMU was determined so as to check the physical stability of the prepared bio-flexy films in high moist conditions. Bio-flexy films of 1cm diameter were kept in saturated solution of aluminium chloride in desiccator. The humidity inside the desiccator was maintained at 79.5%. Removed the films after 3 days, weighed and calculated percentage moisture absorption.
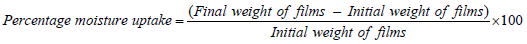
In vitro drug release study of formulations: Performed by using Modified M.S. Apparatus) [9,10]. Buffer pH 7.4 was filled in 36 vials (receiver compartment). These were kept in thermostatically controlled compartment. Tied egg membranes to donor compartment (containing formulations). Donor compartments were inserted into receiver compartments. Temperature was kept constant at 37°C with orbital shaker incubator. Sampling was done at regular intervals from 10 min to 48 hours. Buffer was completely replaced after every sampling. Performed Ultra Violet Spectral analysis of every sample.
Stability studies of prepared films: Stability studies of prepared films were conducted as per ICH Guidelines. Stability testing of pharmaceutical product is done to ensure the efficacy, safety and quality of active drug substance and dosage forms and shelf life or expiration period. The stability studies of the formulations were performed at 40°C ± 2°C with ± 45 ± 5% RH, at 25 ± 2°C with 60 ± 5% RH and at 2 ± 5°C conditions of temperature and relative humidity for 3 months. Observed for change in pH, Folding Endurance and in vitro drug release of formulations [11-13].
Isolation of biomaterial
The isolated biomaterial from pulp of Solanum melongena showed % yield of 0.58% ± 0.02.
Physicochemical properties of isolated biomaterial (Table 3)
| S. No. | Physical properties | Characteristics |
|---|---|---|
| 1 | Colour | Brown |
| 2 | Odour | Odourless |
| 3 | Taste | Tasteless |
| 4 | Texture | Powder |
| 5 | Colour changing point | 186 ± 2°C |
| 6 | Solubility | Acetone |
Table 3: Physical properties of isolated Solanum melongena biopolymer.
Chemical properties (Table 4)
| S. No. | Chemical properties | Test | Characteristics observed | Inference |
|---|---|---|---|---|
| 1 | Carbohydrates | Molisch reagent test | Appearance of purple colour at interface of 2 layers | Carbohydrates were present |
| 2 | Proteins | Biuret test | Appearance of violet colour | Proteins were present |
| 3 | Starch | Iodine test | An intense blue black colour appeared | Starch was present |
| 4 | Reducing sugar | Fehling’s test | Brick red precipitate appeared | Reducing sugar was present |
Table 4: Chemical properties of isolated Solanum melongena biopolymer.
Topiramate-Solanum melongena biopolymer interaction studies:
a) Dry method λmax at 752 nm showed no significant difference with that of pure Topiramate.
b) Wet method λmax at 752 nm showed no significant difference with that of pure Topiramate. Therefore, drug-excipient interaction did not occur (Table 5).
| Potassium | Crystal | Iodine | Copper | Potassium | Methyl | Methyl orange | Ferrous sulphate |
|---|---|---|---|---|---|---|---|
| Permanganate | Violet | Sulphate | Dichromate | Red | |||
| Pink to brown colour change | Purple | Light brown | Blue | Light colour | Red | Brown | Light brown |
| change |
Table 5: Drug polymer interaction with different reagents.
Colorimetry
To 0.05 g of Topiramate, 0.05 mL each of crystal violet, iodine, copper sulphate, potassium permanganate, potassium dichromate, ferrous sulphate, methyl orange, methyl red were added on different areas of glass plate. Colour change of drug from pink to brown occurred with potassium permanganate. This depicted reaction of potassium permanganate because of double bonds saturation. Similarly, Drug (0.05 g)+Polymer (0.05 g) also revealed similar colour change with potassium permanganate. Thus the drug was not entrapped. U.V. analysis showed λmax of Topiramate-Solanum melongena biopolymer mixture near to that of pure Topiramate. Thus, there was no interaction between drug and biomaterial and isolated Solanum melongena biopolymer was found to be compatible with Topiramate and useful as bio-excipient in formulating nanosized drug loaded bio-flexy films. Drug+Biopolymer showed no colour change with other reagents.
Spectral studies of the isolated bio-materials
Infra-red spectroscopy: The result of Infra-red spectra of isolated Solanum melongena biopolymer showed peaks at 1619cm-1, 1638cm-1, 1117cm-1, 3131 cm-1, 1319cm-1 that showed inbuilt mucoadhesive property due to functional groups, RCONH2, RNH2, RCOOH, C=CCOOH, S=O (Figure 2).
DSC: Peak was obtained at 116.94°C (Figure 3).
NMR Spectral analysis: The presence of proton and their environment was confirmed by 1HNMR. The 1HNMR Spectra confirms the presence of carbohydrates residue within the biopolymer extracted as the shift of carbohydrate ring protons are 3-6 ppm and the spectra when compared reflects the peak at 3.5531 ppm (Figure 4).
Preparation of calibration curve of drug: Calibration curve of Topiramate was prepared in Buffer (pH 7.4) showed linearity. Regression square value (R2) was found to be 0.9945 (Figure 5).
Evaluation of prepared formulations
a) Mucoretention time by dynamic method: (Table 6)
| S. No. | Formulation | Dislodgement time (min) | Formulation | Dislodgement time (min) |
|---|---|---|---|---|
| 1 | FMO1 (1:1) | 60 | FSO1 (1:1) | 15 |
| 2 | FMO2 (1:3) | 120 | FSO2 (1:3) | 20 |
| 3 | FMO3 (1:5) | 180 | FSO3 (1:5) | 30 |
| 4 | FMO4 (1:6) | 210 | FSO4 (1:6) | 45 |
| 5 | FMO5 (1:10) | 240 | FSO5 (1:10) | 60 |
Table 6: Dynamic method.
b) Mucoadhesion study by static method: (Table 7)
| S. No. | Formulation | Dislodgement time (min) | Formulation | Dislodgement time (min) |
|---|---|---|---|---|
| 1 | FLO1 (1:1) | 120 | FSO1 (1:1) | 30 |
| 2 | FLO2 (1:3) | 150 | FSO2 (1:3) | 60 |
| 3 | FLO3 (1:5) | 180 | FSO3 (1:5) | 90 |
| 4 | FLO4 (1:6) | 240 | FSO4 (1:6) | 120 |
| 5 | FLO5 (1:10) | 300 | FSO5 (1:10) | 150 |
Table 7: Static method.
c) Swelling percentage study: Swelling Percentage Study of bio-flexy films containing Solanum melongena biopolymer (FMO1-FMO5) ranged from 70% ± 0.5 to 73% ± 0.3. Swelling increased on increasing biopolymer content. It indicates that drug released through bio-flexy films by swelling followed by erosion.
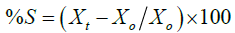
Where, Xt=Final Weight of swollen bio-flexy film after time t
Xo=Initial Weight of bio-flexy film at time zero
Thickness of prepared films: Nanosized Topiramate loaded Solanum melongena bio-flexy films (FMO1-FMO5) showed Thickness of 0.027 ± 0.006 mm to 0.037 ± 0.002 mm.
Tensile strength of bio-flexy films: Tensile Strength of bio-flexy films containing Solanum melongena biopolymer (FMO1-FMO5) was in range from 89.38 g ± 2.8 to 100.05 g ± 2.4. This shows prepared bioflexy films can withstand any undue pressure or strain.
Folding endurance of films: Folding endurance of formulations FMO1-FMO5 was found to be 102-167.
Surface pH of formulations: Surface pH of nanosized Topiramate-Solanum melongena bio-flexy films (FMO1-FMO5) was 7.01 ± 0.05 to 7.01 ± 0.03. Thus films were optimal for soft palatal delivery being in physiological soft palatal pH range.
Uniformity of weight: Prepared Nanosized Topiramate-Solanum melongena biopolymer bio-flexy films FMO1-FMO5 showed weight uniformity of 0.020 ± 0.05 to 0.031 ± 0.04.
Uniformity of drug content: Prepared Nanosized Topiramate-Solanum melongena biopolymer bio-flexy films FMO1-FMO5 showed drug content uniformity of 90.4% ± 0.65 to 98.6% ± 0.60. This indicates that drug was uniformly dispersed in bio-flexy films.
Percentage moisture uptake (PTU) of bio-flexy films: Formulations FMO1-FMO5 showed Percentage Moisture Uptake (PTU) of 2.2% ± 0.13 to 2.6% ± 0.12.
In vitro release study: In vitro release Study by Modified M.S. apparatus showed FMO1 (Nanosized Topiramate: Solanum melongena biopolymer bio-flexy film in ratio of 1:1) as Best Formulation as shown in Figure 6 (comparative graph of in vitro Drug Release of Formulations FMO1 to FMO5 by Modified M.S. Apparatus) and Figure 7 (comparative graph of in vitro Drug Release of Formulations FSO1 to FSO5 by Modified M.S. Apparatus).
The rationale of research work was to explore a novelistic route for brain specifically via Trans Soft Palatal route. The potential of nanosized Topiramate loaded bio-flexy films for Trans-Soft palatal to brain drug delivery was investigated. Biopolymer was isolated from natural edible sources of Solanum melongena pulp. Isolated biopolymers was preferred as bio-excipient for preparation of nanosized drug loaded bio-flexy films as it possessed in-built properties such as filmability, mucoadhesivity, mucoretentivity, non-toxicity, biocompatibility, biodegradability, inertness, non-reactiveness to soft palate. Topiramate-Solanum melongena biopolymer interaction did not occur. Biopolymer was inert, lacked irritancy on soft palate and thus was suitable for preparing formulations. Bio-Flexy films were formulated by Film Solvent Casting method. Prepared formulations showed prolonged effect for up to 48 hours. A smart conclusion was drawn that the isolated biopolymer possessed in-built filmability and mucoadhesive properties and served as bio-excipients for formulating nanosized drugs loaded bio-flexy films. Soft palatal drug delivery bypasses oral route and reduces economic and API dose burden in patients. As bio-flexy films were prepared using biopolymer, it is more economic and safer than synthetic polymers used in marketed formulations. Bio-flexy films are easy to administer and remove/ discontinue from site of administration. Soft palate is non-keratinized, devoid of salivary enzymes so minimal drug wastage occurs. 5 ratios of Nanosized Topiramate: Solanum melongena biopolymer; FMO1 (1:1), FMO2 (1:3), FMO3 (1:5), FMO4 (1:6), FMO5 (1:10) were prepared, and similarly 5 ratios of Nanosized Topiramate: Sodium CMC (Sodium Carboxyl Methyl Cellulose; FSO1 (1:1), FSO2 (1:3), FSO3 (1:5), FSO4 (1:6), FSO5 (1:10) were prepared. Films were formulated by Film Solvent Casting method. Various Evaluation parameters were performed for prepared formulations. The isolated Solanum melongena biopolymer showed percentage yield of 0.58% ± 0.02. Prepared formulations were more mucoretentive on Capra aegagrus intestinal mucosa than standard Sodium Carboxyl Methyl Cellulose films. Formulations FMO1-FMO5 possessed Swelling Percentage: 70% ± 0.5 to 73% ± 0.3, Thickness: 0.027 ± 0.006 mm to 0.037 ± 0.002 mm, Tensile Strength: 89.38 g ± 2.8 to 100.05 g ± 2.4, Folding Endurance: 102-167, Surface pH: 7.01 ± 0.05 to 7.01 ± 0.03, Weight Uniformity: 0.020 ± 0.05 to 0.031 ± 0.04, Drug Content Uniformity: 90.4% ± 0.65 to 98.6% ± 0.60, Percentage Moisture Uptake (PTU): 2.2% ± 0.13 to 2.6% ± 0.12.
Formulation FMO1 (containing Topiramate: Solanum melongena biopolymer (1:1)) flexy film was found to be Best Formulation as per all the Evaluation parameters. It showed R2 = 0.9618, Higuchi Matrix as best fit model, follows Anomalous Transport release mechanism, T50%: 24 hours, T80%: 32 hours using BITS Software 1.12 (Table 8 and Table 9). Stability study revealed stable bio-flexy films with no significant change in physical appearance and stable pH. Prepared formulations of Topiramate loaded bio-flexy films containing Solanum melongena biopolymer were suitable for Soft Palatal Delivery (Tables 10 and 11).
| S. No. | Formulation | Dislodgement time (min) | Formulation | Dislodgement time (min) |
|---|---|---|---|---|
| 1 | FLO1 (1:1) | 120 | FSO1 (1:1) | 30 |
| 2 | FLO2 (1:3) | 150 | FSO2 (1:3) | 60 |
| 3 | FLO3 (1:5) | 180 | FSO3 (1:5) | 90 |
| 4 | FLO4 (1:6) | 240 | FSO4 (1:6) | 120 |
| 5 | FLO5 (1:10) | 300 | FSO5 (1:10) | 150 |
Table 8: Time required for 50% of drug to release (T50%) and time required for 80% of drug to release (T80%) values of topiramate-Solanum melongena polymer bio-flexy films.
| Ratio | T50% (hours) | T80% (hours) |
|---|---|---|
| FSO1 (1:1) | 27 h | 48 h |
| FSO2 (1:3) | 24 h | 48 h |
| FSO3 (1:5) | 26 h | 31 h |
| FSO4 (1:6) | 27 h | 32 h |
| FSO5 (1:10) | 26 h | 31 h |
Table 9: Time required for 50% of drug to release (T50%) and time required for 80% of drug to release (T80%) values of Topiramate-Sodium carboxyl methyl cellulose flexy films.
| In vitro release study kinetics of Nanosized Topiramate-Solanum melongena biopolymer bio-flexy films | |||||||
|---|---|---|---|---|---|---|---|
| Formulations | R2 | Best fit equation/model | Mode of action | ||||
| Zero order | First order | Higuchi matrix | Peppas | Hixon Crowell | |||
| Kinetics | Kinetics | Equation | Model | Model | |||
| FMO1 (1:1) | 0.8307 | 0.8311 | 0.9618 | 0.9172 | 0.831 | Higuchi matrix equation | Anomalous transport |
| Mechanism | |||||||
| FMO2 (1:3) | 0.5395 | 0.5408 | 0.9244 | 0.8816 | 0.5404 | Higuchi matrix equation | Fickian diffusion |
| (Higuchi matrix) mechanism | |||||||
| FMO3 (1:5) | 0.7835 | 0.784 | 0.9436 | 0.8846 | 0.7838 | Higuchi matrix | Fickian diffusion |
| Equation | (Higuchi matrix) mechanism | ||||||
| FMO4 (1:6) | 0.788 | 0.7888 | 0.9031 | 0.9591 | 0.7885 | Peppas Korsmeyer | Fickian diffusion |
| Model | (Higuchi matrix) | ||||||
| Mechanism | |||||||
| FMO5 (1:10) | 0.7392 | 0.7403 | 0.8996 | 0.9515 | 0.7399 | Peppas Korsmeyer | Fickian diffusion |
| Model | (Higuchi matrix) mechanism | ||||||
Table 10: In vitro release study of Nanosized Topiramate-Solanum melongena biopolymer bio-flexy films.
| In vitro release study kinetics of Nanosized Topiramate-Sodium CMC (Sodium carboxyl methyl cellulose) standard polymer flexy films | |||||||
|---|---|---|---|---|---|---|---|
| Formulations | R2 | Best fit equation/model | Mode of action | ||||
| Zero order | First order kinetics | Higuchi matrix equation | Peppas | Hixon Crowell | |||
| Kinetics | Model | Model | |||||
| FSO1 (1:1) | 0.917 | 0.9172 | 0.9311 | 0.961 | 0.9171 | Peppas | Fickian diffusion (Higuchi matrix) Mechanism |
| Korsmeyer | |||||||
| Model | |||||||
| FSO2 (1:3) | 0.8454 | 0.846 | 0.8947 | 0.9009 | 0.8458 | Peppas | Fickian diffusion (Higuchi matrix) Mechanism |
| Korsmeyer | |||||||
| Model | |||||||
| FSO3 (1:5) | 0.9049 | 0.9051 | 0.9425 | 0.9698 | 0.9051 | Peppas | Anomalous transport mechanism |
| Korsmeyer Model | |||||||
| FSO4 (1:6) | 0.8963 | 0.8963 | 0.9319 | 0.9566 | 0.8963 | Peppas | Fickian diffusion (Higuchi matrix) mechanism |
| Korsmeyer | |||||||
| Model | |||||||
| FSO5 (1:10) | 0.8989 | 0.8989 | 0.9371 | 0.9614 | 0.8989 | Peppas | Anomalous transport mechanism |
| Korsmeyer Model | |||||||
Table 11: In vitro release study of Nanosized Topiramate-Sodium CMC (Sodium carboxyl methyl cellulose) standard polymer flexy films.
In this study, Bio-flexy films of nanosized Topiramate were prepared with isolated Solanum melongena biopolymer along with co-processing agents. Evaluation parameters were performed ad compared with that of Standard Flexy Films of Nanosized Topiramate-Sodium CMC (Sodium Carboxyl Methyl Cellulose) Flexy Films. This research work explores the potentiality of Oro-Trans Soft Palatal route as novel drug delivery platform along with suitability of isolated Solanum melongena biopolymer in comparison with standard polymer. Results showed that prolonged drug action can be achieved by this Soft Palatal drug delivery route for up to 48 hours. Formulation FMO1 (containing Topiramate: Solanum melongena biopolymer (1:1)) was found to be Best Film. This study acts as an alternative therapy for treatment of convulsions in low dose, sub-minimal side effects, economic to patients and manufacturers.
We wish to acknowledge Prof. K.K. Raina (Vice Chancellor, DIT University) and Mr. Anuj Aggarwal, (Chairman, DIT University) for providing the platform for research.
The authors declare that there is no conflict of interest.