
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Case Report - (2021)Volume 11, Issue 3
Acetaminophen is a common toxic ingestion and the leading cause of acute liver failure in the United States. There exists a relative paucity of evidence in guiding management of acetaminophen Extended-Release ingestions compared to immediate-release ingestions. Many case reports involving Extended-Release acetaminophen ingestion are confounded by co-ingestion, resulting in a delayed peak acetaminophen level. Few reports have been published involving pure acetaminophen Extended-Release ingestion with a late crossing of the Rumack-Matthew nomogram. We present a case of witnessed isolated acetaminophen Extended-Release ingestion in an 18-year-old male, resulting in an acetaminophen level crossing the Rumack-Matthew nomogram treatment threshold at 21 hours post-ingestion.
Acetaminophen; Toxic; Ingestion
Acetaminophen (APAP) is one of the most commonly consumed analgesics in the United States. It is also the most common cause of acute liver failure in the United States [1]. APAP is a weak acid (pKa ≅ 9.5), and at physiologic pH, it is rapidly absorbed from the duodenum [2]. Acetaminophen Extended-Release (APAP ER) is a formulation of acetaminophen often with an ethylcellulose polymer coating used to extend the half-life of the drug and decrease dosing requirements [3]. Studies have found the use of this polymer coating can increase the half-life of APAP ER by approximately 157% (4.02 hours vs. 2.56 hours for APAP ER and immediate release, respectively [4]. The half-life of APAP is dynamic and increases when consumed at toxic levels compared to therapeutic levels [2]. There is a paucity of data regarding the management of isolated overdose of Acetaminophen Extended- Release (APAP ER). Several case studies have been published about patients who have overdosed on Acetaminophen Extended-Release (APAP ER), but they are complicated by co-ingestion [5,6]. Further, it is not clear how coingestions affect absorption of APAP ER, thus, the management of APAP ER overdose remains problematic. The Rumack-Matthew nomogram was not designed or validated for the plotting of serial concentrations to determine hepatotoxicity, nor was it designed for use with Acetaminophen Extended-Release (APAP ER). For Immediate Release (IR) acetaminophen, it is well known that a single four-hour level will determine if treatment with n-acetylcysteine is necessary [7-9]. With APAP ER, the treatment threshold is not clear. We present a case of a basic military trainee who overdosed on Acetaminophen Extended-Release (APAP ER) and did not cross the Rumack-Matthew nomogram treatment threshold until 21 hours post-ingestion.
An 18-year-old male active duty military trainee took 24 tablets of 650 mg (240 mg/kg) Acetaminophen Extended-Release (APAP ER), as witnessed by his roommate. As an active duty military trainee the patient lived in a closely monitored environment. Trainees only have access to prescribed medications, and have no access or ability to purchase over the counter medications. Further, trainees are accompanied by their roommate at all times and to all locations. The patient denied ingesting any other medications, which was verified by his roommate. The patient presented to the emergency department 1.5 hours after ingestion and was given 75 g of activated charcoal. A 4-hour post-ingestion acetaminophen level of 140 ug/mL was obtained (Figure 1).
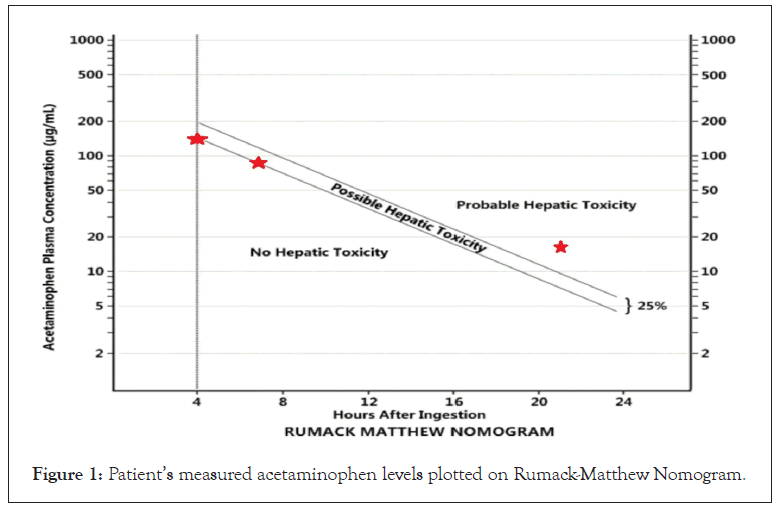
Figure 1: Patient’s measured acetaminophen levels plotted on Rumack-Matthew Nomogram.
Treatment with n-acetylcysteine was initiated and serial acetaminophen levels were obtained. The 7-hour post-ingestion acetaminophen level was 89 ug/mL, just below the treatment threshold of 89.18 ug/mL. However, the patient’s 21-hour level was 16 ug/mL, above the treatment threshold of 7.88 ug/mL. This patient’s acetaminophen level crossed the Rumack-Matthew treatment line at a level drawn 21 hours after ingestion. The patient had received N-acetylcysteine since 4 hours post-ingestion and experienced no transaminase elevation. He was admitted to the hospital and recovered uneventfully.
Other published cases of Acetaminophen Extended-Release (APAP ER) overdose that resulted in crossing the treatment line on the Rumack-Matthew nomogram are often complicated by co-ingestion of other substances [6]. This case is an example of a pure APAP ER overdose resulting in a 4 hour level below the treatment line, but a delayed 21 hour level above the treatment line. Prior case series have shown that delayed peaks in serum acetaminophen levels are relatively uncommon, with one study showing zero of 41 patients having a delayed peak in serum Acetaminophen (APAP) levels [10]. Acetaminophen Extended-Release (APAP ER) is absorbed over an extended period of time and when Acetaminophen Extended-Release (APAP ER) was introduced, the question was raised if this could cause delayed toxicity. In 1996, this issue was investigated by having research participants take 75% of a toxic dose of immediate release Acetaminophen (APAP), and then their serum acetaminophen levels were trended. These same participants repeated the experiment one week later using the same dose of Acetaminophen Extended-Release (APAP ER). None of the participants went on to cross the treatment threshold after a negative four-hour level. This study concluded that a single fourhour level is sufficient to detect overdoses with Acetaminophen Extended-Release (APAP ER) [11]. Another study recommends checking an initial level and then a repeat level 4-6 hours after the initial level, assuming it was drawn 4-8 hours after ingestion. If both Acetaminophen (APAP) levels are below the treatment line, treatment was thought to be unnecessary [10].
Our case is an example of one where our patient did not cross the treatment threshold until 21 hours after ingestion. It is possible that our patient had taken significantly more Acetaminophen Extended-Release (APAP ER) than other studies causing the delayed crossing of the treatment line. To our knowledge, this is one of the first case reports of an isolated Acetaminophen Extended-Release (APAP ER) overdose exhibiting the late crossing of the Rumack- Matthew treatment line. Interestingly, Drs. Roberts and Buckley published a study in 2008 where their patient had taken a large dose of Acetaminophen Extended-Release (APAP ER) (1.2 g/ kg). Their patient’s maximum serum concentration occurred at approximately 20 hours post-ingestion [12].
While our patient’s Acetaminophen (APAP) level crossed the treatment line, it is unknown if they would have suffered liver injury without n-acetylcysteine. Additionally, the impact of the activated charcoal upon the serum Acetaminophen (APAP) concentration is unknown. Further investigation is necessary to determine if a single, initial 4-hour acetaminophen concentration is sufficient to identify all patients requiring treatment with n-acetylcysteine to prevent liver injury.
Citation: Inman BL, Tannenbaum L, Maddry JK, Bridsong S (2021) Delayed Toxicity in an Isolated Extended-Release Acetaminophen Ingestion: A Case Report. J Clin Toxicol. 11:483.
Received: 29-Mar-2021 Accepted: 12-Apr-2021 Published: 19-Apr-2021 , DOI: 10.35248/2161-0495.21.11.483
Copyright: © 2021 Inman BL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.