Gynecology & Obstetrics
Open Access
ISSN: 2161-0932
ISSN: 2161-0932
Research Article - (2015) Volume 5, Issue 9
Objectives: The aim of this study was to investigate whether the partition-defective 3 protein (Par3) regulate cervical carcinoma growth and metastasis.
Methods: Immunohistochemistry were used to analyze the expression of Par3 protein in samples from 89 cervical squamous cell carcinoma (CSCC) patients among Uighur women. The specific short hairpin (shRNA) vector as well as eukaryotic expression vector of PARD3 was transfected into SiHa cell lines. The variation of migration and invasion after transfection was detemined using Transwell assays, cell cycle and apoptosis were assayed by flow cytometry, respectively.
Results: The incidence of CSCC was associated with reduced expression of Par3. Down-regulation of Par3 was significantly associated with more advanced tumors (i.e., higher histological grade, lymph node involvement, and higher tumor stages) (p<0.05 for all). Lost expression of Par3 promotes proliferation, inhibits apoptosis, and enhances migration and invasion. Loss of Par3 induces MMP9 expression and epithelial to mesenchymal transition (EMT) related genes (N-cadherin, E-cadherin and β-Catenin) expression changed in SiHa cells.
Conclusions: The reduced Par3 expression in cervical cancer indicating tumor-suppressive properties of Par3 that may be a marker of poor prognosis in cervical cancer patients and the molecular determinants of epithelial polarity which have tumorigenesis enhancing impact might through EMT.
Keywords: Par3 protein; Cervical cancer; Epithelio-mesenchymal transition; MMP9; RNAi
Globally, cervical cancer is the third most common cancer among women with an estimated 528,000 new cases and 266,000 deaths in 2012 [1]. It have been confirmed that High-risk human papillomavirus (HPV) infection is the principal risk factor for the development of cervical cancer. Therefore, it is one of the few preventable human cancers and its prevention is based on the early diagnosis of precancerous lesions [2]. However, in developing countries, cervical cancer remains the most common cause of cancer-related deaths due to the inadequate support and even though 80% of cervical cancer cases occur in these countries [3]. In Xinjiang, a region in west China, cervical squamous cell carcinoma (CSCC) is considered a major public health problem and is the most commonly occurring cancer among Uyghur women [4]. Although considerable improvements achieved through systemic therapy, the prognosis of cervical cancer patients with recurrent or metastasis stills unfavorable. Therefore, a tumor metastasis associated markers is required to develop effective treatments for cervical cancer.
Cell polarity is one of the most basic properties of all normal cells and it is essential for maintenance of tissue homeostasis, which regulated by multiple polarity proteins including Par3, Par6, and atypical protein kinase C (aPKCs) that form a Par complex [5,6]. Among this Par complex, Par3 is a multi-modular scaffold protein that encoded by polarity protein PAR-3 gene (PARD3) which interacts with diverse cell polarity regulators to control cell signaling and it is necessary for the establishment of apico-basal polarity [7]. Recently, it has been assumed that loss of polarity and disruption of cell junctions to be a key step of epithelial-derived cancer cells, and increasing evidence also suggests that such defects play a direct role in the pathology of cancer [8], which indicating that loss in tissue architecture and oncogenesis may go side by side. The loss in structure associated with malignant transformation is likely to be involved changes in the expression, localization, and activation patterns of key polarity proteins. Furthermore, loss of cell polarity and cell–cell adhesion was commonly observed in advanced tumors and correlates strongly with their invasion into adjacent tissues and the formation of metastasis [9-11].
However, recent studies showing that Par3 also display prooncogenic activities in hepatocellular carcinoma and renal cell carcinoma [12,13] suggest a more complex regulation of the polarity machinery during cellular transformation. Thus, Par3 proteins may be involved in multiple aspects of oncogenesis because a relationship exists between polarity dysfunction and cancer progression. However, whether polarity protein regulates cervical cancer growth and metastasis is poorly understood. Here, to elucidate a potential role of the polarity protein in oncogenic processes of cervical cancer, we focused on polarity protein Par3 belong to the apicobasal cell polarity machinery family, analyzed the association of cancer development with altered gene expression at protein levels. Par3 expression silenced in the SiHa cervical cancer cell lines and determined whether loss of Par3 drives tumor growth and metastasis.
Ethics statement
All patients and controls provided written informed consent, and we received study approval from the ethics committee of the First Affiliated Hospital of Xinjiang Medical University.
Patient samples
We obtained cervical tissue specimens from Uighur women with CSCC and from those who did not have cervical diseases but received hysterectomies in the Department of Gynecology at the First Affiliated Hospital in Medical University of Xinjiang. All cancers were staged in accordance with the criteria established by the International Federation of Gynecology and Obstetrics (FIGO). Formalin-fixed, paraffin-embedded (FFPE) tissues (n=89) were obtained from the Department of Pathology. FFPE specimens or fresh-frozen cervical tissues were collected during an initial outpatient visit, during gynecologic examination, or after a surgical procedure involving general anesthesia. Tumor samples were collected within 30 minutes (min) of surgical resection. None of the patients received chemotherapy or radiation prior to surgery. After evaluation by a pathologist, tumor tissues were immediately frozen in liquid nitrogen and stored at -80°C. Haematoxylin and eosin staining was also performed to confirm the diagnosis and to analyze pathological grades, metastasis, and tumor cell content. Seventy percent of all tumor samples were composed of tumor cells free of necrosis.
Patients included 54 FIGO stage I B and 35 FIGO stage IIB. There were 38 well-differentiated cases, 23 moderately differentiated and 28 poorly differentiated tumors. Lymph node metastasis was documented in 31 patients. The median age of patients with cervical cancer was 49.5 years (IQ range 28–65.5 years). Control tissues (n=66) were from patients who did not have cervical lesions or cancer but had hysterectomies for other reasons (i.e., fibroids, prolaps uteri, adenomyosis, or a combination of fibroids with prolaps uteri) during the same time period.
Immunohistochemistry (IHC)
IHC staining was performed with an anti-Par3 rat monoclonal antibody (1:300 Abcam, Cambridge, MA, USA). Sections (3-mm-thick) were cut from paraffin-embedded tissue blocks. Samples were dewaxed in xylene and rehydrated in alcohol and distilled water. Antigen retrieval was then performed by heating samples for 15 min at 95ºC in citrate buffer (pH 6.0). Samples were cooled to room temperature and incubated in 3% hydrogen peroxide to quench peroxidase activity. After incubating at 4ºC overnight in primary antibody and washing with Tris buffer, biotin-labeled secondary antibody was added for 15 min followed by streptavidin peroxidase for 15 minutes. After eluting with PBS, diaminobenzidine and haematoxylin counterstaining were performed.
Two pathologists evaluated the percentage and intensity of staining in tumor cells in a blinded manner. The pathologists reached a consensus number for each tumor sample. cytoplasmic Par3 were quantified according to intensity (0, 1+, 2+, or 3+) and percentage (0%–100%) of staining.
Cell culture and transfections
SiHa cells (ATCC; Manassas, VA, USA), a human cervical squamous cell carcinoma cell line, were cultured in RPMI 1640 plus 10% calf serum and 1% penicillin/streptomycin in a 5% CO2 humidified incubator at 37°C. SiHa cells were seeded in six-well plates and grown to 60%-80% confluence. Par3 in the eukaryotic expression vector pcDNA3.1 (5- TCCGCTCGAGATGA TGGACTTGGAGCTGCC-3, antisense 5- ATGGGGTACCGAG TTTTTCTTAACATCTGGC-3), NRF2 inhibitor (10620318-267429 G04/10620318-267429 F12), and the scrambled sequence (UUCAAAGUCACCUC GGCAACUGCGG/ CAACAGCUGGC UUCCUCAAHCAGAA) were synthesized by Invitrogen (ShangHai, CN). Transfection complexes were formed with Lipofectamine RNAiMAX (Invitrogen, CA, USA) in Opti-MEMI (Invitrogen, CA, USA) according to manufacturer guidelines. Negative controls were cultured in normal conditions. All transfections were performed in triplicate. Cell proliferation was determined by counting cells 24, 48, and 72 hours (h) after transfection. RNA and protein were extracted 48 h or 72 h, respectively, after transfection.
RNA isolation and qRT-PCR
We isolated total RNA using Trizol reagent (Invitrogen, CA, USA) per manufacturer’s instructions. RNA was reverse transcribed into cDNA using the Prime-ScriptTM one-step qRT-PCR kit (C28025- 032, Invitrogen). Pard3 forward primer: 5’-CAGGTGCATCGC TTGGAAC-3’, reverse primer 5’-GCTGAGACATTG TTGGTGCC-3’. All samples used SYBR Select Master Mix (Applied Biosystems, TX, USA). We evaluated β-Actin expression for normalization. Relative gene expression was determined with the comparative delta-delta CT method (2 -ΔΔCt). Reaction mixtures for PARD3 analyses were incubated at 95°C for 10 min and 40 cycles at 95°C for 15 seconds followed by 60°C for 1 minute. We evaluated β-actin at 95°C for 10 min and 40 cycles at 95°C for 15 seconds followed by 55°C for 1 minute.
Protein isolation and western blotting
Protease inhibitors (Boster, Wuhan, China) were also added to cell lysates, which were maintained on ice for 20 minutes. Lysates were then centrifuged at 12,000 rpm for 10 min at 4°C. Samples (50 μg) were boiled for 5 min in sample buffer and then separated on 12% gels by SDS-PAGE. Gels were transferred onto nitrocellulose membranes and blocked for 1 h in 5% skim milk at room temperature with shaking. Primary antibodies for rabbit anti- MMP9, anti-TIMP2, anti-β1 integrin, anti-E-cadherin, anti-β-Catenin, anti-vimentin, anti-N-cadherin and anti-Par3 (Abcam, USA) or β-Actin (Sangon, Shanghai, China) was added overnight to blots at 4ºC. Blots were washed in PBS-Tween three times, after which the secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G; Thermo, IL, USA) was added at room temperature for 2 hours. Chemiluminescent substrate (Thermo, IL, USA) was added to visualize bands. Quantity one software was used to quantify the intensity of each band and was normalized to the intensity of the internal control β-Actin. Results were expressed as fold changes normalized to control values.
Analyses of cell cycle and apoptotic changes by flow cytometry
SiHa cells were seeded in six-well culture plates at a density of 5×104 cells/well in RPMI 1640 plus 10% calf serum and 1% penicillin/ streptomycin. High-fucose-content (HFC) polysaccharide (50, 100, 200, or 250 μg/mL) was added for 1 h followed by the treatment with 300 μM H2O2 for varying time points (0–24 h). Cell cycle distributions were examined by measuring PI-fluorescence with a BD FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA, USA) through an FL-2 filter (585 nm). We recorded 1×104 events per sample. Data were analyzed with Cell Quest.
Annexin V staining was performed to evaluate apoptosis. Control and treated SiHa cells were added at 5×105 cells/mL in binding buffer (10 mM HEPES [(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] (pH 7.4), 140 mM NaCl, 2.5 mM CaCl2). FITC-annexin V (10 μl) in 190 μl of cell suspension was incubated for 10 min at room temperature. Cell mixtures were centrifuged and resuspended in 190 μl binding buffer, and 10 μl PI (1 mg/mL) solution was added. Cells were acquired on a FACS Calibur flow cytometer at 1×104 events per sample. Necrotic cells were defined as positive for both PI and annexin V and were excluded from further analysis.
Transwell migration and invasion assays
Migration and invasion assays were performed as previously described. Migration was evaluated in Transwell cell culture chambers with 6.5 mm-diameter polycarbonate membrane filters containing 8-μm pores (Corning, NY, USA). Cells were added in 100 ml serumfree media to the upper chamber. The lower chamber contained 600 ml culture media with 10% calf serum. After 10 h at 37ºC, cells were removed from the upper surface of the membrane with a cotton swab. Filters were fixed in methanol for 20 min and stained with Giemsa solution for 30 minutes. We then counted the number of cells that had migrated. Five random fields (Nikon ECLIPSE TS100) were counted per well, and the mean was calculated. The membrane of the upper chamber of the transwell was pre-coated with 100 ml of a 1 mg/ml solution of Matrigel (BD, USA).
Statistical analysis
Statistical analyses were determined using SPSS Version 17. P values were two-sided, and the significance level was P<0.05. Values were expressed as means ± SEM. Statistical analyses were conducted using the two-tailed Student’s t-test upon verification of the assumptions. Mann-Whitney test was used to test continuous variables for differences in Par3 IHC scores between tumor and normal tissues. In addition, we performed Spearman’s tests for correlations.
Loss of Par3 promotes cell proliferation and apoptosis in SiHa cells
To examine if Par3 has a potential function in cervical carcinogenesis, we used PARD3-specific short hairpin (shRNA) or a full-length human PARD3 to transfect SiHa cells and to determine if the promotion of tumor growth by change expression of Par3.The transfection efficiency was as high as 89.7%. Transfection efficiency of SiHa cells expressing PARD3-shRNA was assessed by flow cytometry (Becton–Dickinson, Franklin Lakes, NJ, USA). Expression of Par3 was detected by real-time quantitative PCR and Western blotting. Both Par3 mRNA and protein levels were significantly decreased after transfecting PARD3-shRNA compared with the vector control and normal groups. Conversely, Par3 expression was significantly increased after transfecting pCDNA3.1·PARD3 (Figure 1).
Figure 1: The detection of Par3 protein transfection with mimics and inhibitor Morphology of transfected Siha cells for 48 h under microscopy (magnification ×200). A. Transfection with Short interfering RNA (SiRNA); B. transfection with mimics; C. The levels of Par3 protein detected by Western blotting after transfection for 72 h.1 and 2 were normal control; 3 and 4 were Knockdown group; 5 and 6 were normal control; 7 and 8 were overexpression group; D The relative expression of Par3 was displayed, which normalized to b-tubulin. There is a statistically significant difference between the group transfected with Par3 mimics, Par3 inhibitor and normal control. **P < 0.01.
Using flow cytometry analysis investigates proliferation and apoptosis rates of Siha cells after altered Par3 expression (Figure 2). The percentage of SiHa cells in G0/G1 phase significant decreased (32.43% ± 1.50%) 48 h after PARD3 knockdown compared with the percentage of control cells in G0/G1 (53.97% ± 2.89%). As can be seen in Figures 4(c) and Table 1, in SiHa cells, lost expression of Par3 significantly induced cell cycle arrest at the S phase. More specifically, the percentage of cells at the S phase increased from 41.97% ± 3.70% for untreated cells to 64.43% ± 2.47% for cells treated with PARD3 siRNA. Over-expression of Par3 significantly increased the percentage of cells in G0/G1 at 48 h (70.20% ± 3.25%) compared with that of control (53.57% ± 2.86%). The percentage of PARD3-transfected cells in S phase decreased (25.07% ± 2.28%) compared with control (44.50% ± 2.35%, Table 2). These results suggest that Loss expression of Par3 increased the basal proliferation rates and promotes DNA replication of the Siha cell lines. There were 1.20% ± 0.36% of SiHa cells that demonstrated apoptotic changes 48 h after PARD3 knockdown; this was a significant decrease compared with control (2.68% ± 0.38%, Table 3). By contrast, over-expression of Par3 was significantly increased apoptosis (8.07% ± 0.71%) compared with 3.60% ± 0.50% in control (Table 4).
Figure 2: Lost expression of Par3 positively modulates CSCC cellular malignant phenotypes. A, D, G and J: Cell apoptosis, Proliferation, Migration and invasion in Siha cells, respectively (Normal controls). B, E, H and K: a Knockdown of Par3 decreased cell apoptosis, enhanced cell proliferation, migration and invasion, which significantly increased malignant phenotypes of Siha cells. C, F, I and L: Overexpression of Par3 sharply increased cell apoptosis, decreased cell proliferation, migration and invasion, which significantly inhibited cell proliferation and migration in Siha cell line. All experiments were performed at least three times.
| G0/G1(%) | S(%) | G2/M(%) | |
|---|---|---|---|
| control | 54.40 ± 3.91 | 42.23 ± 3.12 | 3.37 ± 2.87 |
| Negative control | 53.97 ± 2.89 | 41.97 ± 3.70 | 4.10 ± 0.87 |
| PARD3 G04 | 47.37 ± 0.72 | 49.60 ± 1.47 | 3.37 ± 0.51 |
| PARD3 F12 | 32.43 ± 1.50Δ | 64.43 ± 2.47Δ | 3.13 ± 0.97 |
Note: ≥ compared with control group, P<0.01;
Table 1: Changing Siha cells cycle after Pard3 Sirna vector transfect 48 hours ( 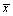 ± 3,N=3).
± 3,N=3).
| G0/G1(%) | S(%) | G2/M(%) | |
|---|---|---|---|
| Control | 53.40 ± 2.16 | 43.80 ± 1.04 | 2.80 ± 1.25 |
| Negative control | 53.57 ± 2.86 | 44.50 ± 2.35 | 1.93 ± 0.51 |
| PcDN3.1 PARD3 | 70.20 ± 3.25Δ | 25.07 ± 2.28Δ | 1.73 ± 0.99 |
Note: ≥ compared with control group, P<0.01;
Table 2: Changing Siha cells cycle after pcdn3.1 pard3 vector transfect 48 hours ( 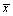 ± 3,N=3).
± 3,N=3).
| Groups | Apoptosis rate of Siha cell(%) |
|---|---|
| control | 2.93 ± 0.67 |
| Negative control | 2.678 ± 0.38 |
| PARD3 G04 | 2.27 ± 0.21 |
| PARD3 F12 | 1.20 ± 0.36Δ |
Note: ≥compared with control group, P<0.01;
Table 3: Changing apoptosis rate of Siha cell lines in response to altered Par3 expression by transfect pard3 Sirna vector after 48 hours (  ± 3,N=3).
± 3,N=3).
| Groups | Apoptosis rate of Siha cell(%) |
|---|---|
| control | 3.30 ± 0.46 |
| Negative control | 3.60 ± 0.50 |
| PcDN3.1 PARD3 | 8.07 ± 0.71Δ |
Note: ≥compared with control group, P<0.01;
Table 4: Changing apoptosis rate of Siha cell lines in response to altered Par3 expression by transfect Pcdn3.1 Pard3 vector after 48 hours ( ± 3,N=3).
Par3 acts as a migration and invasion suppressor in SiHa cells
Invasive growth is an important biological characteristic of malignant cancer cells. To determine if change expression of Par3 infect the cell motility, we performed a Transwell assay in Siha cells. The results show that Cell migration abilities was inhibited after Par3 over-expression compared with control (Table 5), and Siha cells with reduced expression of Par3 were enhanced the migration ability (Table 6). Overexpression of Par3 decreased the invasive abilities of Siha cells (Table 7). As expected, Siha cells with reduced expression of Par3 were more invasive compared with control cells (Table 8). These results suggest that Par3 promotes migration and invasion in SiHa cells.
| Groups | Count of SiHa cells |
|---|---|
| Control | 194.00 ± 33.85 |
| Negative control | 222.75 ± 29.09 |
| PcDN3.1 PARD3 | 78.60 ± 14.26Δ ∇ |
Note: Δcompared with control group,P<0.05; ∇compared with Negative control group,P<0.05;
Over expression of Par3 inhibit Siha cells migration.
Table 5: Changing Siha cells migration ability after Pcdn3.1 Pard3 vector transfect.
| Groups | Count of SiHa cells |
|---|---|
| Control | 159.00 ± 7.55 |
| Negative control | 170.67 ± 12.86 |
| PARD3 G04 | 282.00 ± 7.48Δ ∇ |
| PARD3 F12 | 229.25 ± 28.59Δ ∇ |
Note: Δcompared with control group, P<0.01;compared with Negative control group,P<0.01;Suppression of Par3 increases Siha cells migration.
Table 6: Changing Siha cells migration ability after Pard3 Sirna vector transfect.
| Groups | Count of SiHa cells |
|---|---|
| Control | 159.00 ± 7.55 |
| Negative control | 168.00 ± 14.53 |
| PcDN3.1 PARD3 | 94.00 ± 6.56Δ ∇ |
Note: ≥compared with control group,P<0.05; ∇compared with Negative control group,P<0.05;Over expression of Par3 inhibit Siha cells invasion
Table 7: Changing Siha cells invasion ability after Pard3 Sirna vector transfect.
| Groups | Count of SiHa cells | |
|---|---|---|
| Control | 159.00 ± 7.55 | |
| Negative control | 170.67 ± 12.86 | |
| PARD3 G04 | 282.00 ± 7.48Δ ∇ | |
| PARD3 F12 | 229.25 ± 28.59Δ ∇ | |
| Note: ≥compared with control group, P<0.01;compared with Negative control group,P<0.01;Suppression of Par3 increases Siha cells invasion | ||
Table 8: Changing Siha cells invasion ability after Pard3 Sirna vector transfect.
Loss of Par3 induces MMP9 expression and epithelial to mesenchymal transition (EMT) related gene expression changed in SiHa cells
Tumor migration often requires expression of matrix metalloproteinases (MMPs), which degrade the extracellular matrix. Epithelia-mesenchymal transition (EMT) enables epithelial cells to acquire migratory potential with concomitant severing of cell–cell contacts and cellular polarity. To test whether loss of Par3 would alter MMP expression, or expression of other epithelial to mesenchymal transition (EMT) related gene, western blot analysis of MMP9, E-cadherin, β-Catenin and N-cadherin following PARD3 siRNA treatment in SiHa cervical cancer cells.
As illustrated in Figure 3, the level of MMP9, a member of the matrix metalloproteinases family, increased remarkably in SiHa cells following the PARD3 siRNA treatment. Expression of proteins involved in EMT also changed in different levels. The level of N-cadherin protein, which is present in mesenchymal cells and expression of N-cadherin make the cells motile increased remarkably following the PARD3 siRNA treatment. However, the level of E-cadherin protein, a calcium-dependent cell-surface glycoprotein encoded by the CDH1 gene is important for maintaining epithelial cell-cell adhesion, cellular polarity differentiation, growth, cell migration, decreased in PARD3 siRNA-treated cells compared to negative control. The level of β-Catenin protein that formation the adhere junction with E-cadherin decreased remarkably following the PARD3 siRNA treatment. These results suggested that loss of Par3 inhibited E-cadherin junction stability, disrupted membrane and actin dynamics at cell-cell junctions and decreased cell-cell cohesion. The upregulated of MMP9 was also helps to degrade the basement membrane to facilitate the movement of migratory phenotype, due to MMP9 also known as collagenase IV, is highly expressed by epithelial cells undergoing EMT.
Figure 3: Loss of Par3 induces MMP9 expression and epithelial to mesenchymal transition (EMT) related gene expression changed in SiHa cells 1 and 2 Expression of MMP9, E-cadherin, β-Catenin and N-cadherin in Siha cells, respectively. 3 and 4 expression of MMP9, E-cadherin, β-Catenin and N-cadherin proteins in PARD3 siRNA treated SiHa cells.
Par3 expression in female Uighur patients with cervical cancer
To address the relevance of Par3 loss to human cervical cancer, we explored the expression of the Par3 protein in tumors from selected female Uighur patients by immunohistochemical staining. Antibodies were tested on formalin-fixed, paraffin-embedded, normal cervical tissues and CSCC. Par3 was mainly localized in the cytoplasm of normal cervical epithelial cells (Figure 4). Significant reductions in Par3 expression were apparent in invasive poorly differentiated carcinomas compared to normal cervical epithelia tissue (P<0.05). Moreover, Par3 was loss expressed in 31 Lymph node metastasis CSCC specimens (Table 9, P<0.05). These results suggest that lost expression of Par3 is important not only for SiHa cell lines but also for the tumorigenic properties of primary cervical cancers.
Figure 4: Detection of Par3 protein expression assessed by immunohistochemical staining in representative specimens of normal cervical epithelia and CSCC, respectively. A: Expression of Par3 in normal cervical epithelia with Strong cytoplasm staining; B weak expression of Par3 proteins in CSCC tissue; (original magnification, × 200).
| Characteristics | N | Par3 ( -) | Par3 ( +) | P |
|---|---|---|---|---|
| Normal mucous epithelia | 66 | 16 | 50 | |
| CSCC | 89 | 61 | 28 | 0 |
| Differentiation | ||||
| well | 38 | 16 | 22 | |
| Moderate | 23 | 18 | 5 | |
| Poor | 28 | 27 | 1 | 0.002 |
| L/N metastasis | ||||
| Negative | 58 | 36 | 22 | |
| Positive | 31 | 25 | 6 | 0.003 |
| FIGO Stage | ||||
| ≤ I B | 54 | 31 | 23 | |
| > IIB | 35 | 30 | 5 | 0.011 |
Table 9: Statistical analysis of Par3 expression and clinicopathologic factors in cervical cancer.
Normal cell polarity signaling is crucial for maintenance of tissue integrity and disordered epithelial cell polarity may contribute to development of EMT and this process related to the EMT is a key step in tumorigenesis [14]. Cervical cancer arises from epithelial cells that have acquired changes in cell apical/basal polarity and proliferation capacity. In the current study, we focus solely on the roles of Par3 in cervical cancer development and intended to associate Par3 related molecular findings in cervical cancer with EMT that is important during the progression of tumor cells to metastatic stages, and to discuss the potential mechanisms underlying the biological functions of Par3.
Our results show that Par3 protein levels were markedly reduced in primary cervical cancer compared with normal control, and this reduced expression of Par3 protein was significantly associated with invasive poorly differentiated carcinomas, and positive lymph node metastasis. The result is consistent with report that reduced Par3 expressing human breast cancer is at significant risk of cancer progression and mortality [15,16]. In addition, similar reports have suggested by Zen et al. [17,18], whereby the deletion and reduced expression of Par3 promote the progression of esophageal squamous cell carcinoma. And exogenous expression of PARD3 gene in PARD3-deficient esophageal squamous cell carcinoma KYSE30 and KYSE270 cell lines enhanced the recruitment of zonula occludens-1 (ZO-1), a marker of tight junctions to cell-cell contact sites. However, knockdown of PARD3 caused a disrupted localization of ZO-1 protein at cell-cell borders. Florencia et al. [19] also demonstrate HPVs induce the loss of cell polarity in that HPV E6 oncoprotein is able to bind and induce the mislocalisation of Par3 protein in a PDZ-dependent manner without significant reduction in Par3 protein levels. In addition, E6 protein promotes a delay in tight junction formation when analyzed through calcium switch assays. The result suggested that the Par3 regulates invasion and metastasis in cancers by controlling tight junction assembly.
We also investigated the functional role of Par3 in a cervical cancer cell line. Proliferation of SiHa cells was enhanced, apoptosis was significantly inhibited, and migration and invasion were enhanced after knockdown PARD3 gene expression. Cells were effective in triggering S phase arrested in the cell cycle after PARD3 siRNA treatment. S phase is defined as the specific period during the cell cycle when DNA synthesis is taking place, resulting in a double quantity of DNA per cell. Conversely, proliferation was inhibited; apoptosis was promoted, and migration and invasion were inhibited after over-expression of Par3. Several studies suggest that a primary function of Par3 is to act as a scaffold or a hub protein that recruits the other proteins. Par3 was shown to regulate apical/basal polarity and regulate protrusive activity through RHOA degradation that mediated by CDC42, Par3 mediates cell protrusion by interaction with Tiam1 and regulates RAC GTPases activity, while RHO, RAC and CDC42 proteins that belonging to the family of small GTPases are critical regulators of migration Activation of Par3/ Tiam1 complex was proved to be the main event at the apical of polarized migrating cells [20,21]. Girdin is an actin-binding protein that regulates migration of various cells. Par3 physically interacts with Girdin, and Girdin together with the Gαi3 controls tight junction formation, apical domain development and actin organization downstream of Par3 [22,23]. Additionally, Par3 could increase bradykinin receptor interactions with PLC1, which also catalyze the activation of PLC downstream of heterotrimeric G proteins [24-26]. Therefore, the Par3 resemble the central cellular machinery for generating apical/basal polarity axis during cell migration.
An important characteristic of invasive cancer cells is the induction of epithelial- mesenchymal transition (EMT), and interestingly, one of the features of EMT is the loss of polarity that acquired by deregulation of transcription program of polarity related genes [27]. In this study, to determine if the promotion of tumor growth by loss of Par3 is related to altered EMT-related genes expression, we analyzed the E-cadherin, β-Catenin and N-cadherin protein expression after PARD3 siRNA treatment in SiHa cervical cancer cells. Notably, overexpression of N-cadherin protein or reduced expression of E-cadherin, β-Catenin is commonly found in PARD3 siRNA treated SiHa cells. Here it may be noted that lost expression of Par3 plays an important role in the induction of EMT. The EMT is an important phenotypic switch that enables cancer cells to migrate and invade. During EMT, the down regulation of E-cadherin causes the release of β-catenin in the cytoplasm, which translocates to the nucleus and the cells undergoing transition express N-cadherin which is mesenchymal markers and try to acquire migratory potential. alter the cell surface protein that promotes epithelial link to neighboring cells and the basement membrane by N-cadherin provides more transient adhesive capability, thus preparing the cell for the gain of motile mesenchymal phenotypes, and thereby conferring a more migratory and invasive properties [28-30]. Our data demonstrate that loss of expressions of both E-cadherin and β-catenin along with down-regulation of Par3 was significant in the context of EMT. EMT signaling including Integrin, TGFβ and JAK/Stat3, small GTPases such as RAS and GF-mediated signaling through PI3K also appears to regulate Par3 during polarity establishment and cell migration [31,32]. The molecular mechanism of Par3 as a regulator of JAK/Stat3 signaling pathways to induce EMT has successfully been investigated in invasive breast cancer [15]. TGFβ induced phosphorylation of polarity protein on the conserved serine 345 is also implicated in EMT in breast cancer [33]. This finding raised the question of the EMT pathway induced by Par3 on SiHa cells, prompting further investigation.
MMP9 is a mumble of zinc-dependent proteins that degrade components of the extracellular matrix and play a major role in tumor invasion and metastasis. Here, loss of Par3 induction of MMP9 expression in PARD3 siRNA treated SiHa cells. It may be noted that down regulation of Par3 denoted impairment in epithelial tight junctions and concomitant N-cadherin expression acquire migratory potential, and add to the up regulation of MMP9 the disruptor of basement membrane component and permitting escape from the primary tumor formation of invasion spots.
In summary, we identified a tumor-suppressive property function of Par3 in human cervical cancer and the lost Par3 expression may be a marker of poor prognosis in cervical cancer. The target of Par3 proteins might have important consequences during the progression of cervical lesions; however, further investigations need to be undertaken to determine EMT pathway induced by Par3 on cervical cancer cells.
This study was supported with The Academy of Sciences Foundation of China (YBXM-2014-06). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.