Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Research Article - (2025)Volume 16, Issue 8
Background: Reperfusion therapy is one of the main methods for alleviating myocardial infarction. However, this treatment may cause injury to the myocardium, known as Hypoxia/Reoxygenation (H/R) injury. Dapagliflozin is a kind of novel hypoglycemic drug, but its role in modulating the H/R injury of cardiomyocytes has not been fully clarified.
Methods: The H/R model and MI rats were established. Reactive Oxygen Species (ROS) level was examined by 2’, 7’- Dichlorofluorescein-Diacetate (DCFH-DA) assay. The release of Lactate Dehydrogenase (LDH) and Creatine Kinase- MB (CK-MB) were examined by enzyme-linked immunosorbent assay. Cell viability was measured by MTT assay and the cell apoptosis was validated by flow cytometry. MicroRNA-124-3p (miR-124-3p), Signal Transducer and Activator of Transcription 3 (STAT3), Phosphoinositide 3-Kinase (PI3K), phospho-PI3 Kinase (p-PI3K) and phosphorylation- STAT3 (p-STAT3) expressions were measured by quantitative Real-Time PCR (qRT-PCR) or Western blot. Besides, dual-luciferase reporter gene assay was performed to confirm the interaction between miR-124-3p and STAT3. Echocardiography was performed to assess cardiac function.
Results: Dapagliflozin could greatly decrease the levels of ROS, LDH and CK-MB, promote cell viability and inhibit the apoptosis; dapagliflozin up-regulated miR-124-3p expression and down-regulated STAT3, p-STAT3 and inactivate the PI3K/AKT pathway. miR-124-3p inhibition or STAT3 overexpression counteracted the effects of dapagliflozin on ROS, LDH and CK-MB levels and the viability and apoptosis of AC16 cardiomyocytes. Dapagliflozin treatment reduced the dilatation and contractile dysfunction of left ventricle in MI rats.
Conclusion: Dapagliflozin protects cardiomyocytes from H/R injury through modulating miR-124-3p/STAT3 and PI3K/AKT axis, suggesting it may be a promising drug for treating heart ischemia-reperfusion injury.
Hypoxia reoxygenation; Cardiomyocytes; Dapagliflozin; STAT3; miR-124-3p
Cardiovascular disease is the most prevalent factor leading to death worldwide, with Acute Myocardial Infarction (AMI) being one of the most common diseases and reperfusion therapy after the onset of AMI symptoms is essential to improve the survival rate and reduce infarct size; paradoxically, reperfusion can induce the death of cardiomyocytes, eventually leading to an increase in infarct size and additional injury, which is called Myocardial Ischemia-Reperfusion Injury (MIRI). Therefore, indepth study of the mechanism of MIRI pathogenesis and exploration of new therapy targets for MIRI are important, which may improve the prognosis with AMI and other cardiovascular diseases.
In recent year, the safety and efficiency of Sodium-Glucose Cotransporter 2 (SGLT2) inhibitor in reducing the level of blood glucose have been gradually validated and the mechanism is mediating glucose reabsorption in renal proximal tubule.
Interestingly, multiple studies have suggested that SGLT2 inhibitor, can also exert cardioprotective effects. For instance, dapagliflozin is a kind of SGLT2 inhibitor and in patients with heart failure, it ameliorates the death of cardiomyocytes and block disease progression. Importantly, application of dapagliflozin has become the category I recommendation option for therapy of patients with heart failure and reduced ejection fraction. However, the mechanism by which dapagliflozin exerts its cardioprotective effect is unknown, especially in MIRI [1,2].
As reported, microRNAs (miRNAs) feature prominently in regulating the pathogenesis of cardiovascular diseases. Among them, miR-124-3p is a promising diagnostic marker and therapeutic target for a variety of cardiovascular diseases. For example, inhibiting miR-124-3p alleviates AMI by blocking the apoptosis of cardiomyocyte and the release of inflammatory factors through regulating NKRF/NF-κB pathway. MiR-124-3p expression is greatly inhibited in myocardial tissue of MIRI rats and the restoration of miR-124-3p counteracted Hypoxia/ Reoxygenation (H/R)-induced cardiomyocyte apoptosis and the release of inflammatory factors such as TNF-α, IL-6 and IL-1β. However, the mechanism by which miR-124-3p attenuates MIRI has not been fully revealed.
In this study, with an in vitro H/R model and myocardial infarction rat model, we report that dapagliflozin protects cardiomyocytes from MIRI by regulating the miR-124-3p/Signal Transducer and Activator of Transcription 3 (STAT3) axis and Phosphatidylinositol 3-Kinase (PI3K)/Akt pathway in vitro H/R model and reduces the dilatation and contractile dysfunction of left ventricle caused by I/R injury in vitro H/R model. This study provides experimental basis for applying dapagliflozin in clinical treatment of MIRI.
Cell culture
Human cardiomyocyte cell line AC16 cells were available from American Type Culture Collection (ATCC, Rockville, MD, USA) and cells were routinely cultured in Dulbecco's Modified Eagle's Medium (DMEM) (thermo-fisher Scientific, Waltham, MA, USA) with 10% Fetal Bovine Serum (FBS) (thermo-fisher scientific), 100 U/ml penicillin (thermo-fisher scientific) and 100 μg/ml streptomycin (thermo-fisher scientific) at 37°C in 5%CO2 in a humidified incubator. Besides, the cells in the logarithmic growth phase were trypsinized by 0.25% trypsin and passaged (thermo-fisher scientific, Waltham, MA, USA) every 3-4 d [3].
Cell transfection
MiR-124-3p mimics, miR-124-3p inhibitors and their corresponding negative controls (miR-NC and Inh-NC), STAT3 overexpression plasmid (STAT3) and blank plasmid (vector) were synthesized by GenePharma Co., Ltd. (Shanghai, China). AC16 cells in the logarithmic growth phase were cultured to 50-60% confluence. Next, the above-mentioned oligonucleotides/plasmids were transfected into AC16 cells with lipofectamineTM 2000 (thermo-fisher scientific, Waltham, MA, USA) according to the manufacturer’s instructions. After an additional 48 h of culture, cellular RNA was subsequently extracted, with the transfection efficiency examined by quantitative Real-Time Polymerase Chain Reaction (qRT-PCR).
Establishment of H/R model
AC16 cells were cultured in a closed incubator with glucose-free and serum-free DMEM (thermo-fisher scientific) for 12 h with oxygen concentration less than 1%, followed by reoxygenation (21%) for different times (1, 2, 4, 8 h) to construct the H/R model. AC16 cells without H/R treatment were used as controls.
Dapagliflozin (purity of 98%) was available from national institute for the control of pharmaceutical and biological products (Beijing, China) [4]. Dapagliflozin was dissolved in normal saline and diluted by serum-free medium, with its concentration adjusted to 5, 10 and 20 μM as the working concentrations. AC16 cells were treated with different concentrations of dapagliflozin prior to H/R treatment.
qRT-PCR
Total RNA from AC16 cells was extracted by a TRIzol kit (invitrogen, carlsbad, CA, USA) as protocols, with RNA concentration and purity detected by a spectrometer. Subsequently, RNA was reverse transcribed into cDNA with one step prime script miRNA cDNA synthesis kit (Takara, Dalian, China). PCR was performed on an ABI7500 FAST real-time PCR (thermo fisher scientific, Waltham, MA, USA) with SYBR green mix (Promega, Madison, WI, USA). Relative miR-124-3p expression was calculated by the 2(-ΔΔCt) method and U6 was adopted as an internal reference. The specific primer sequences were: miR-124-3p: forward: 5'-ACA GGCTAAGGCTCCCAGTGAA-3'; reverse: 5'-CGCAGGGTCCGAGGTATTC-3'; U6: forward: 5'- GCTTCGGCAGCACATATACTAAAAT-3'; reverse: 5'- CGCTTCACGAATTTGCGTGTCAT-3'.
Detection of Reactive Oxygen Species (ROS)
The level of intracellular ROS was measured with a ROS assay kit (Beyotime, Shanghai, China) according to the manufacturer’s instruction. Briefly, AC16 cells were transferred into a 96-well plate and accordingly incubated with serum-free medium containing 2',7'-Dichloro Dihydrofluorescein Diacetate (DCFHDA, 10 μM) for 20 min at 37°C. Next, a microplate reader was used to detect the fluorescence intensity of the cells [5].
Enzyme-Linked Immunosorbent Assay (ELISA)
The activity of Lactate Dehydrogenase (LDH) and Creatine Kinase-MB (CK-MB) released by AC16 cells were measured by corresponding ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instruction.
MTT assay
Cardiomyocytes were inoculated in 96-well plates at 2 × 103 cells/well and cultured in an incubator at 37°C in 5% CO2 and 100% humidity for 24 h. Next, 50 μl of MTT solution (5 mg/ mL, Beyotime Biotechnology, Shanghai, China) was added into each well and the cells were then incubated for 4 h at 37°C and after that, the supernatant was accordingly aspirated. Besides, 150 μL of Dimethyl Sulfoxide (DMSO) (Beyotime, Shanghai, China) was added to each well and then the plate was shaken on a shaker to dissolve the formazan and then the OD value of each well was determined by a microplate reader at a wavelength of 570 nm.
Flow cytometry for apoptosis measurement
The degree of apoptosis of cardiomyocytes was examined with an Annexin V-FITC/PI apoptosis detection KIT (Yeasen Biotech Co., Ltd., Shanghai, China) according to the manufacturer’s instruction. The cells in each group were trypsinized and centrifuged, washed twice with cold PBS and then resuspended with binding buffer to a final concentration of 1 × 106 cells/mL. 100 μL of cell suspension was mixed with 5 μL of AnnexinVFITC staining solution and 5 μL of PI staining solution and incubated in darkness at ambient temperature for 15 min. Then the cells were detected with a FACSCalibur flow cytometer (BD Biosciences, SanJose, CA, USA). Ultimately, the apoptosis rate of the cells was analyzed by FlowJo software (BD Biosciences, San Diego, CA, USA).
Dual-luciferase reporter gene assay
Wild Type (WT) and Mutant Type (MUT) luciferase reporter plasmids (STAT3 WT and STAT3 MUT) were synthesized by GenePharma Co., Ltd. (Shanghai, China). STAT3 WT or STAT3 MUT and miR-124-3p mimics or miR-NC were cotransfected into AC16 cells with LipofectamineTM 2000 (thermo-fisher scientific, Waltham, MA, USA). The luciferase activity was accordingly rated by the dual-glo luciferase assay system (Promega, Madison, WI, USA) 48 h after transfection according to the manufacturer’s instruction [6].
Western blot
The medium was discarded and the cells were washed by PBS. Next, AC16 cells were lysed with RIPA lysis buffer (Roche, Basel, Switzerland) on ice for protein extraction and then the protein concentration was quantified with a BCA kit (thermo-fisher scientific, Waltham, MA, USA). For each sample, 20 μg of total protein was accordingly loaded on sodium dodecyl sulfate polyacrylamide gel and electrophoresed and then electrotransferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). Next the membrane was blocked with 5% skimmed milk for 1 h at ambient temperature. Next, the membranes were washed three times for 10 min each time in TBST and incubated overnight at 4°C with primary antibodies (anti-STAT3 antibody, ab32500, Abcam, UK, 1:1000; anti-p-STAT3 antibody, ab267373, Abcam, UK, 1:1000; antip- PI3K antibody, #17366, cell signaling technology, USA, 1:1000; anti-PI3K antibody, #4292, cell signaling technology, USA, 1:1000; GAPDH, ab8245, Abcam, UK). After washing again in TBST, the membranes were incubated with secondary antibody (goat anti-rabbit IgG, ab205718, 1:3000) for 1 h at room temperature. The membranes were rinsed three times with TBST, each time for 10 min. Next, the the protein bands were developed by a ECL kit (Millipore, Bedford, MA, USA) and the gray values of the protein bands were analyzed by ImageJ (NIH, Bethesda, MD, USA). During this experiment, all antibodies were available from Abcam (Shanghai, China).
Determination of cardiac functional parameters
The Left Anterior Descending (LAD) coronary artery was ligated in male SD rats weighing 230-240 g to establish MI models. In order to avoid intestinal obstruction, rats were fasted for 12 hours prior to the operation. A supine position was adopted for all animals after they were anesthetized with isoflurane inhalation. In this study, rats were intubated using a smallanimal ventilator (R415, RWD Life Science Co., Ltd., Shenzhen, China), followed by an MI with ligation of the LAD coronary artery. Using a sterile 5-0 silk suture, a ligature was placed below the left atrial appendage. Ischemic changes shown on the electrocardiogram suggested that the left ventricular myocardium had been successfully ligated and the chest cavity was then closed. The control group underwent similar procedures, except that the LAD was ligated. An anti-infective treatment was administered for three days to all operated animals for a period of 24 hours. This study involved the use of 18 animals. The rats were randomly divided into three groups (n=6), in which they were divided into three groups (control, MI and MI+DAPA). A cardiac function assessment was performed four days following surgery. After being anesthetized with isoflurane, the animals were placed in a supine position. An echocardiographic image was acquired using a Vevo 770 microimaging system equipped with a 25-MHz probe (Visual Sonics, Toronto, ON, Canada). In the parasternal long and short axis views at the level of the papillary muscles, parameters were obtained based on the M-mode and twodimensional images obtained in the M-mode and twodimensional views.
Statistical analysis
All of the experiments were performed in triplicate and SPSS 23.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis in this study. Student’s t-test was executed to compare the means between two groups and one-way analysis of variance with post-hoc test was adopted to compare the means among multiple groups. Statistically, P<0.05 is significant.
Establishment of in vitro H/R model of cardiomyocytes
First of all, we constructed a H/R model with AC16 cells and measured ROS levels in AC16 cells and the results showed that ROS level was gradually increased with increasing reoxygenation time compared with the control (Figure 1A). ELISA showed that LDH and CK-MB levels were greatly increased after H/R treatment (Figure 1B, C). Furthermore, MTT assay suggested that the viability of AC16 cells was markedly reduced after H/R treatment in a time-dependent manner (Figure 1D). Flow cytometry analysis displayed that the proportion of apoptotic AC16 cells was increased markedly after H/R in a timedependent manner (Figure 1E). The above results indicated that the H/R injury model was successfully constructed.
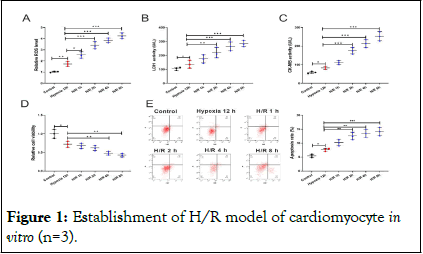
Figure 1: Establishment of H/R model of cardiomyocyte in vitro (n=3).
Note: A) ROS levels of AC16 cells after 12 h of hypoxia at different reoxygenation times were measured by DCFH-DA assay. B, C) LDH and CK-MB levels in AC16 cell supernatants were detected by ELISA. D) The viability of AC16 cells was detected by MTT assay. E) The apoptosis of AC16 cells was detected by flow cytometry.
Dapagliflozin alleviates the injury in AC16 cells after H/R treatment
From the above results, we found that cell injury was most severe after 8 h of reoxygenation, so we conducted subsequent experiments under this condition. Next, we pretreated AC16 cells with different concentrations of dapagliflozin, respectively. It was demonstrated that, ROS level was decreased with increasing concentrations of dapagliflozin (Figure 2A). The results of ELISA suggested that LDH and CK-MB levels were also significantly decreased with increasing concentration of dapagliflozin (Figure 2B,C). Also, as expected, dapagliflozin treatment demonstrably promoted AC16 cell viability and inhibited the apoptosis in a dose-dependent manner (Figure 2D, E). Considering 20 μM dapagliflozin had the most significant effect, so it was used in the follow-up experiments.
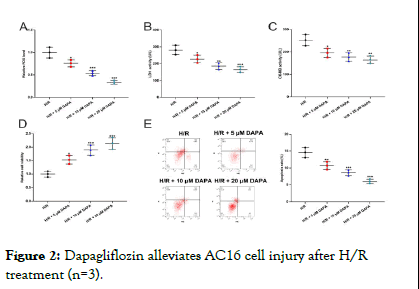
Figure 2: Dapagliflozin alleviates AC16 cell injury after H/R treatment (n=3).
Note: A) ROS levels in AC16 cells treated with different concentrations of dapagliflozin were measured by DCFH-DA assay. B,C) The levels of LDH and CK-MB in the supernatant of AC16 cells after treatment with different concentrations of dapagliflozin was detected by ELISA. D) The viability of AC16 cells after treatment with different concentrations of dapagliflozin was tested by MTT assay. E) The apoptosis in AC16 cells after treatment with different concentrations of dapagliflozin was detected by flow cytometry.
Dapagliflozin attenuates AC16 cell injury by enhancing miR-124-3p expression
To expound the mechanism by which dapagliflozin attenuates AC16 cell injury, qRT-PCR was used to detect the expression of miR-124-3p, miR-130a-3p, miR-17-3p, miR-582-5p and miR-124-3p in AC16 cells. It suggested that miR-124-3p expression was gradually down-regulated with increasing reoxygenation time (Figure 3A). However, miR-124-3p expression was restored after dapagliflozin treatment (Figure 3B, Supplementary Figure 1). However, other SGLT-2i (Ertugliflozin and Canagliflozin) was used and qRT-PCR showed that Ertugliflozin had no effect on the expression of miR-124-3p (Supplementary Figure 2). Subsequently, we transfected miR-124-3p inhibitors into AC16 cells to investigate the role of miR-124-3p in AC16 cells (Figure 3C). DCFH-DA assay showed that, inhibition of miR-124-3p in AC16 cells significantly raised ROS level (Figure 3D). ELISA showed that miR-124-3p inhibition increased LDH and CK-MB levels in AC16 cells (Figure 3E,F). Besides, MTT assay and flow cytometry analysis indicated that miR-124-3p inhibition counteracted the promoting effect of dapagliflozin on AC16 cell viability and the inhibitory effect on its apoptosis (Figure 3G,H).
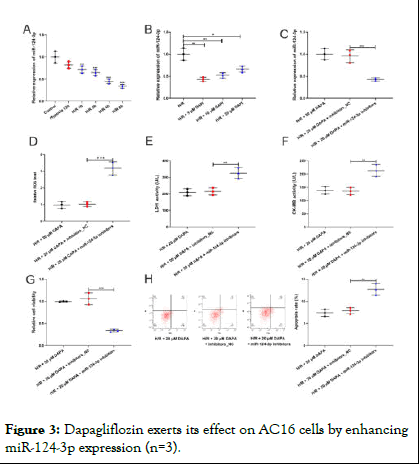
Figure 3: Dapagliflozin exerts its effect on AC16 cells by enhancing miR-124-3p expression (n=3).
Note: A) The expression of miR-124-3p in AC16 cells treated with H/R was determined by qRT-PCR. B) The expression of miR-124-3p in AC16 cells were examined by qRT-PCR after AC16 cells were treated with different concentrations of CA. C) After transfection with miR-124-3p inhibitors, the expression of miR-124-3p in AC16 cells was measured by qRT-PCR. D) After AC16 cells were transfected with miR-124-3p inhibitors and treated with CA, ROS levels were measured by DCFH-DA assay. E,F) The levels of LDH and CK-MB in the supernatant of AC16 cells were measured by ELISA kits. G) The viability of AC16 cells was examined by MTT assay. H) The apoptosis of AC16 cell was analyzed by flow cytometry [7,8].
MiR-124-3p restrains the STAT3 and PI3K-Akt signaling pathway
MiRDB and Genecard databases were used to explore the targeted genes of miR-124-3p and myocardial infarction. A total of 35 genes were selected from the two datasets and STAT3 was selected and the results were shown in Figure 4A. To further research the functions and mechanisms of the identified 35 genes, all genes were assessed by GO term and KEGG analyses. The results of GO analysis demonstrated that the overlapping genes were significantly enriched with response to hypoxia, cell proliferation and regulation of protein phosphorylation in the BP category. Moreover, in the CC analysis, the genes were predominantly involved in extracellular region, membrane raft and cytosol. In addition, receptor-binding, enzyme binding and identical protein binding were remarkably enriched in the molecule function category. Furthermore, the FoxO signaling pathway and PI3K-Akt signaling pathway were significantly enriched in the signal transduction pathway category. The results of the GO and KEGG analyses are shown in Figure 4B and D. Dual-luciferase reporter gene assay showed that miR-124-3p mimics remarkably inhibited the luciferase activity of STAT3 WT, but that of STAT3 MUT was not significantly affected by miR-124-3p (Figure 4C). In addition, we examined the effect of miR-124-3p inhibitors on p-STAT3 and STAT3 expression in AC16 cells with western blot and the results showed that both p- STAT3 expression and STAT3 expression were up-regulated and p-STAT3/STAT3 ratio was markedly increased after transfection of miR-124-3p inhibitors compared with H/R group (Figure 4EF). Besides, the PI3K-Akt signaling pathway was also investigated and the results revealed that DAPA could significantly decrease p-STAT3/STAT3 ratio and miR-124-3p inhibitor upregulated the p-STAT3/STAT3 ratio (Figure 4G). These results suggested that miR-124-3p could probably be a protective factor for cardiomyocytes via repressing STAT3 signaling and PI3K/AKT pathway.
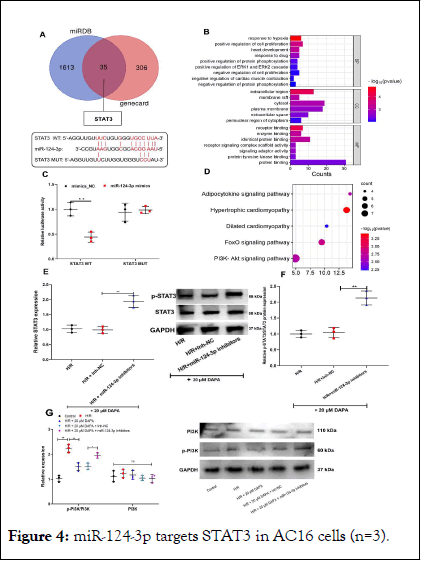
Figure 4: miR-124-3p targets STAT3 in AC16 cells (n=3).
Note: A) The downstream target genes of miR-124-3p were predicted by target scan database and STAT3 was a potential target gene of miR-124-3p. B) The binding relationship between miR-124-3p and STAT3 was verified by a dual-luciferase reporter gene assay. C) The expression of STAT3 in AC16 cells was detected by western blot, after AC16 cells were treated with H/R and transfection of miR-124-3p inhibitors.
Dapagliflozin attenuates the injury of AC16 cell via modulating miR-124-3p/STAT3 axis
Next, we detected STAT3/p-STAT3 expression when AC16 cells were treated with H/R for different times. p-STAT3 expression, STAT3 expression and p-STAT3/STAT3 ratio were remarkably increased with increasing reoxygenation time (Figure 5A). Next, we transfected STAT3 overexpression plasmid and miR-124-3p mimics into AC16 cells, with the transfection efficiency verified by western blot (Figure 5B). The results of ELISA highlighted that miR-124-3p overexpression dramatically reduced the levels of ROS, LDH and CK-MB (Figure 5C-E); however, STAT3 overexpression reversed the above effects (Figure 5C-E). MTT assay and flow cytometry analysis showed that miR-124-3p overexpression remarkably promoted cell viability and suppressed the apoptosis (Figure 5F-G); while STAT3 overexpression reversed the above effects. However, 20 μM dapagliflozin have no effect on the expression of miR-124-3p and STAT3 in AC16 cells under the condition of normoxia (Supplementary Figure 3). Collectively, these results suggest that dapagliflozin pre-treatment attenuates H/R injury of AC16 cells by promoting miR-124-3p expression and down-regulating STAT3.
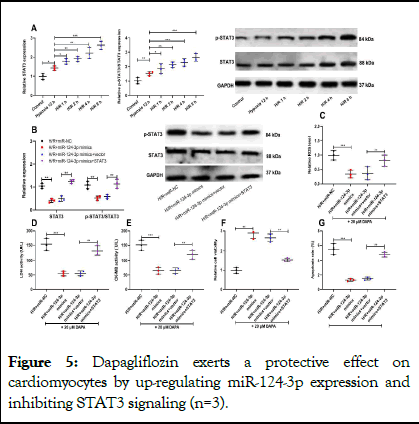
Figure 5: Dapagliflozin exerts a protective effect on cardiomyocytes by up-regulating miR-124-3p expression and inhibiting STAT3 signaling (n=3).
Note: A) The protein of p-STAT3 and STAT3 in AC16 cells treated with H/R were measured by western blot. B) AC16 cells were co-transfected with STAT3 overexpression and miR-124-3p mimics, and then treated with dapagliflozin and the expressions of p-STAT3, STAT3 were measured by western blot. C) The ROS levels of AC16 cells in each group were measured by DCFH-DA assay. D, E) LDH and CK-MB levels in supernatant of AC16 cells in each group were detected by ELISA. F) The viability of AC16 cells in each group was tested by MTT assay. G) Flow cytometry was used to analyze the the apoptosis of AC16 cells in each group.
Dapagliflozin protects the heart from the damage caused by ischemia/reperfusion injury
Dapagliflozin was then studied in order to find out if it had any effect on cardiac function in MI rats. It was determined from echocardiography results that the fractional shortening and the ejection fraction in the hearts of rats that had been injured by I/ R were significantly decreased, whereas administration of dapagliflozin prevented these two values from dropping in the heart of these rats. As a result of dapagliflozin treatment, the increase in left ventricular end-diastolic and end-systolic volumes, as well as left ventricular internal dimensions, was significantly reduced compared to those rats that were not treated with dapagliflozin (Figure 6). The results indicated that dapagliflozin treatment reduced the dilation of the left ventricle and the contractile dysfunction caused by I/R injuries [9,10].
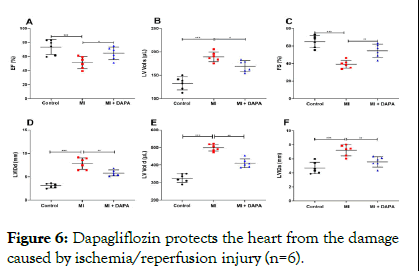
Figure 6: Dapagliflozin protects the heart from the damage caused by ischemia/reperfusion injury (n=6).
Note: A-F) Echocardiography was performed to assess cardiac function.
Dapagliflozin prevents myocardial damage and cell apoptosis through miR-124-3p/STAT3 and PI3K/AKT pathway in cardiomyocytes from myocardial infarction rat model
ROS, LDH and CK-MB levels were markedly increased in cardiomyocytes under the condition of hypoxia and H/R and dapagliflozin significantly decreased the levels of ROS, LDH and CK-MB (Figure 7A). The viability of cardiomyocytes was significantly decreased and the apoptosis rate was decreased in H/R groups compared to the control group. Cell survival rates were significantly improved and cell apoptosis were significantly decreased by 20 μM concentrations of dapagliflozin treatment, respectively (Figure 7B-C). Dual-luciferase reporter gene assay showed that miR-124-3p mimics remarkably inhibited the luciferase activity of STAT3 WT, but that of STAT3 MUT was not significantly affected by miR-124-3p (Figure 7D). Furthermore, dapagliflozin treatment could significantly decrease the STAT3, p-STAT3/STAT3 and p-PI3K/PI3K (Figure 7E).
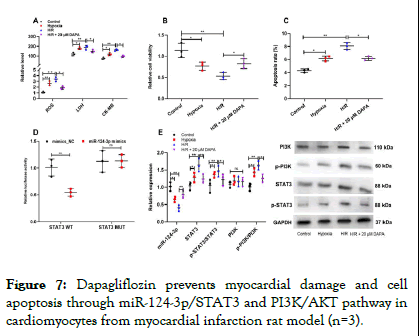
Figure 7: Dapagliflozin prevents myocardial damage and cell apoptosis through miR-124-3p/STAT3 and PI3K/AKT pathway in cardiomyocytes from myocardial infarction rat model (n=3).
Note: A) ROS levels were measured by DCFH-DA assay and LDH and CK-MB levels were detected by ELISA. B, C) Cell viability and apoptosis were measured by CCK-8 and flow cytometry. D) The binding relationship between miR-124-3p and STAT3 was verified by a dual-luciferase reporter gene assay. E) qRT-PCR and Western blot assay were used to measure the expression of miR-124-3p, STAT3, p-STAT3, PI3K and p-PI3K.
Cardiomyocyte apoptosis and inflammatory response have been considered as the main mechanisms of MIRI and recent studies suggest that cardiomyocyte apoptosis is initiated at the beginning of ischemia and is further aggravated after reperfusion. Blocking the process of apoptosis may alleviate myocardial injury caused by ischemia-reperfusion and prevent the occurrence of myocardial stunning and heart failure and many studies have supported that inhibiting the inflammatory response during reperfusion after local ischemic injury is crucial for the recovery of cardiac function. Some previous studies have suggested the potential of dapagliflozin in attenuating the injury of cardiomyocytes induced by multiple factors. Specifically, dapagliflozin reduces cadmium-induced cardiotoxicity via IL6/ STAT3 and TLR2/TNF-α pathways; another study reports that dapagliflozin ameliorates doxorubicin-induced heart injury by regulating oxidative stress and inflammatory response. In the present study, we constructed an in vitro H/R model and in vivo MI model and found that dapagliflozin treatment greatly reduced the levels of myocardial injury markers, promoted cell viability and inhibited the apoptosis, as well as improved cardiac function of MI rats. These results suggests that dapagliflozin can alleviate cardiomyocyte injury resulting from H/R, showing the cardioprotective effects of dapagliflozin, which is consistent with the previous reports.
In cancer biology, miR-124-3p is reported to be a tumor suppressor. For instance, it suppresses the growth and aggressiveness of glioblastoma multiforme by targeting RhoG. In recent years, the biological function of miR-124-3p in cardiovascular disease has been gradually unveiled. A recently published study reports that, miR-124-3p is under-expressed in the heart tissues of a rat model with MIRI and it targets TRAF6 to suppress the inflammatory response and apoptosis of cardiomyocytes. This study reports that miR-124-3p protect cardiomyocytes against MIRI, which is consistent with our demonstrations. We also demonstrated that dapagliflozin pretreatment could up-regulate miR-124-3p in cardiomyocytes, suggesting dapagliflozin may ameliorate MIRI via regulating miR-124-3p/TRAF6 axis. Notably, some other studies report that miR-124-3p promotes the injury of cardiomyocytes via repressing NKRF and SIRT1 in rat model with AMI. These results suggest that miR-124-3p exert distinct roles in the ischemic stage and reperfusion stage.
STAT3 signal transduction pathway is a highly evolutionarily conserved pathway which is involved in cell-to-cell communication, gene transcription regulation, inflammatory response and stress response. STAT3 has been demonstrated to be associated with a variety of cardiovascular diseases, including MIRI, atherosclerosis, cardiac hypertrophy and heart failure. Overwhelming evidence supports that STAT3 has cardioprotective effects. Accumulating studies report that ischemia-reperfusion (or H/R) enhances STAT3 phosphorylation and p-STAT3/STAT3 ratio in cardiomyocytes which is consistent with the findings in the present work. However, in this study, we observed that STAT3 restoration counteracted the cardioprotective function of miR-124-3p. This unexpected results suggest that STAT3 can aggravate MIRI via some non-classical mechanisms in our model, which remain to be clarified in the following work.
Previous work has reported that PI3K/AKT pathway activation is pathogenic in cardiac dysfunction and hypertrophy. Moreover, a study reported that Iva prevented cardiac hypertrophy and fibrosis via inhibiting PI3K/AKT/mTOR pathway in an established transverse aortic constriction mouse model. These findings suggest that the PI3K/AKT pathway-mediated autophagy inhibition is harmful to cardiac homeostasis. Consistently in this work, we demonstrated that the PI3K/AKT pathway was activated during MI, whereas dapagliflozin effectively inhibited PI3K/AKT signaling in H/R induced myocardial cell. Nevertheless, the role of AKT/mTOR signaling in MI progression is quite the opposite according to some other studies.
On all accounts, in this study, through a series of in-vitro and invivo experiments, we demonstrate that dapagliflozin attenuates H/R injury to cardiomyocytes via increasing miR-124-3p. Our study reveals the mechanism of dapagliflozin in alleviating the MIRI and provides experimental basis and new therapeutic strategies for applying dapagliflozin in the clinical treatment of MIRI.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Cen K, Liang X, Ding Y, Yu H (2025) Dapagliflozin Protects Cardiomyocytes from H/R Injury by Regulating miR-124-3p. J Clin Exp Cardiolog. 16:962.
Received: 09-Aug-2024, Manuscript No. jcec-24-33451; Editor assigned: 12-Aug-2024, Pre QC No. jcec-24-33451 (PQ); Reviewed: 28-Aug-2024, QC No. jcec-24-33451; Revised: 03-Sep-2025, Manuscript No. jcec-24-33451 (R); Published: 10-Sep-2025 , DOI: 10.35248/2155-9880.25.16.962
Copyright: © 2025 Cen K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.