
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2015) Volume 5, Issue 1
Objective: Organic extracts from nine species (eleven populations) of the Bursera genus native to Mexico, were investigated for cytotoxic and anti-inflammatory activities. The influence of the cytotoxic plant extracts on cell cycle progression and apoptosis were also analyzed.
Materials and methods: Cytotoxic activity from B. ariensis (Kunth) McVaugh and Rzed. Kew Bull (two populations), B. bicolor Engl., B. lancifolia Engl., B. glabrifolia Engl. (two populations), B. fagaroides (La Llave) Rez., Calderón and Medina, B. linanoe (La Llave) Rez., Calderón and Medina, B. galeottiana Engl., B. kerberi Engl., and B. excelsa Engl. were evaluated by the sulforhodamine B protein staining assay against four carcinoma cell lines: KB (nasopharyngeal), HF-6 (colon), MCF-7 (breast), and PC-3 (prostate), as well as on HFS-30 fibroblast normal skin cell line. Chloroform extracts from B. ariensis (two populations), B. kerberi and B. galeottiana were added to PC-3 cells to analyze percentage of cells in G1/S and G2/M phases by flow cytometric analysis, while apoptosis was determined in a fluorescence microscope. In vivo anti-inflammatory experiments were conducted through the 12-O-tetradecanoylphorbol-13-acetate (TPA) induced ear edema in mice.
Results: With the exception of B. glabrifolia and B. excelsa, hexane extracts of all the collected plant species displayed selective cytotoxic activity in at least one cell line. For the chloroform extracts, only B. bicolor, B. kerberi, B. galeottiana, and B. fagaroides exhibited cytotoxic activity, while for the ethyl acetate extracts B. fagaroides was the only plant displaying cytotoxic effect. There was no cytotoxic activity detected with methanol extracts. While B. galeottiana showed an important effect in G2/M arrest, induced apoptosis in PC-3 cells was detected with B. ariensis (two populations) and B. galeottiana. Important anti-inflammatory activity similar to the control indomethacin was displayed by B. galeottiana, B. excelsa, and B. schlechtendalii.
Conclusion: Findings demonstrated that the studied species possess pharmacological potential qualities for developing plant medicines.
Keywords: Cytotoxic; Bursera ; Anti-inflammatory; Cell cycle; Apoptosis; Ethnomedical use
From the genus Bursera, approximately 80 of the 84 species growing in Mexico are endemic, and are one of the most abundant plant groups of the tropical dry forest [1]. In traditional Mexican medicine several burseras are used in the treatment of cancer, although the term “cancer” includes general conditions consistent with cancer symptomatology, i.e., inflammations, ulcers; and such dermatological conditions as hard swellings, abscesses, calluses, corns, warts and polyps [2]. Since pre-hispanic times “copal”, the bark and resin from Bursera trees, has been employed in the treatment of cancer symptoms, as well as against some other conditions [3-5]. Some examples are the following: B. excelsa known as pomo or tecomahaca [6] is used to treat tumors, and muscle spasms [7]; B. glabrifolia known as zomplante is used to treat fever, inflammation and weakness in Oaxaca [8]; B. galeottiana known as cuajiote colorado is used as an analgesic, healing herb, and to treat abcesses [9]; B. linanoe known as copal is used in the treatment of gums inflammation [10]; the water extract of B. fagaroides known as copalillo is believed to have an anticancer effect [11]; B. bicolor known as ticumaca is used as a medicine to treat inflammation of the muscles [12,13]; B. ariensis known as copal blanco is used to treat cold and a disease called ‘sacar el frío’, which is a term related to inflammation [14], and B. schlechtendalii is used in the treatment of flu [15].
Results of previously conducted experiments on other bursera species demonstrated various biological activities. Against cancer, some of them have displayed cytotoxic action; for example, B. bipinata, B. copallifera and B. fagaroides were toxic against several human cancer cell lines that included nasopharyngeal, colon, breast and prostate carcinomas, while B. graveolens and B. grandiflolia were active against fibrosarcoma and epidermoid carcinoma, respectively [2,16]. Other studies with B. microphylla, B. fagaroides, B. schlechtendalii, B. klugii, and B. morelensis demonstrated antitumor actions against tumors transplanted in mice [11,17-20].
In the present investigation we studied nine Mexican species of the Bursera genus which, as mentioned above, are currently in popular use to treat patients with cancer symptomatology. Using traditional and still currently applied ethnomedical information, we conducted experimental analyses to study cytotoxic and anti-inflammatory activities that tend to validate their popular uses. We also determined the influence of the cytotoxic plant extracts on cell cycle progression and apoptosis.
To our knowledge, the eleven selected populations belonging to nine species have no previously documented investigations, and this new information opens up the possibility of advancing medicinal approaches from these natural Mexican autochthonous resources.
Plant material
Nine Mexican species (eleven plant populations) of the genus Bursera were included in this study. Seven populations were collected in the central part of the Mexican Republic. The species B. ariensis (Kunth) McVaugh and Rzed. Kew Bull., B. bicolor Engl., B. glabrifolia Engl., B. fagaroides (La Llave) Rez., Calderón and Medina, and B. linanoe (La Llave) Rez., Calderón and Medina were collected in the state of Morelos, while B. kerberi Engl. and B. ariensis (Kunth) McVaugh and Rzed. Kew Bull. Were collected in the state of Mexico. Four populations were collected in the southeast of Mexico: B. excelsa Engl. was collected in the state of Chiapas; from Oaxaca B. glabrifolia Engl. and B. galeottiana Engl. were collected, and B. schlechtendalii was collected in Puebla. It is important to point out that for B. glabrifolia two plant populations were collected, one in Morelos, and the other in the southeastern state of Oaxaca where it was noticed on an unplanned trip; as also happen in the case of a second collection for B. ariensis, with one collected in Morelos while the other was fortuitously found on an unplanned trip to Zacazonapan in the state of Mexico. All plant material was collected between September and November 2010. Voucher specimens have been deposited at the Herbarium of the Centro de Investigación en Biodiversidad y Conservación (CIByC), UAEM and were authentified by Dr. J. R. Bonilla-Barbosa (Table 1).
| Botanical name | Local name | Coordinates of collection | Site of collection | Voucher specimen no. |
|---|---|---|---|---|
| B. ariensis | Copal blanco | 18°260’00’’N 99°02’00’’O | Cd. Ayala, Morelos | 27198 |
| B. bicolor | Ticumaca | 18°26’00’’N 99°02’00’’O | Cd. Ayala, Morelos | 27196 |
| B. glabrifolia | Copal | 18°26’00’’N 99°02’00’’O | Cd. Ayala, Morelos | 27200 |
| B. fagaroides | Copalillo | 18°26’00’’N 99°02’00’’O | Cd. Ayala, Morelos | 27199 |
| B. linanoe | Linaloe, Copal | 18°28’00’’N 99°06’00’’O | Tlaquiltenango, Morelos | 27197 |
| B. glabrifolia | Jioteblanco | 16°55’00’’N 96°24’00’’O | Mitla, Oaxaca | 27206 |
| B. galeottiana | Cuajilotecolorado | 16°55’00’’N 96°24’00’’O | Mitla, Oaxaca | 27195 |
| B. kerberi | Copal | 19°05’00’’N 100°15’00’’O | Zacazonapan, México | 27205 |
| B. ariensis | Copalillo | 19°05’00’’N 100°15’00’’O | Zacazonapan, México | 27204 |
| B. excelsa | Pomo,Tecomahaca | 19°05’00’’N 100°15’00’’O | Tuxtla Gutierrez, Chiapas | 27203 |
| B. schlechtendalii | Copal | 18°45’00’’N 100°15’00’’O | Teotlaco, Puebla | 13297 |
Table 1: Collection data of the investigated Mexican Bursera species.
Extraction
All bark of the plant material was air-dried and then pulverized with a grinder. The powdered plant material (10 g) was extracted with 100 mL of n-hexane (3x) in a sonicator for 30 min, and filtered at room temperature. To obtain three other extracts, the same procedure was applied but using first chloroform, then ethyl acetate followed by methanol. All of the extracts were then concentrated under reduced pressure at 40°C and stored at 4°C for later use.
Reagents
RNase, Propidium iodide (PI) and Acridine orange (AO) were obtained from Molecular Probes, Life Technologies, Sigma-Aldrich, MO, USA. RPMI-1640, Fetal bovine serum (FBS), Trypsin- EDTA and NaHCO3 were obtained from In vitrogen-GIBCO, Carlsbad, CA, USA.
Cytototoxic assay
Cytotoxicity of plant extracts was measured by the sulforhodamine B (SRB) (MP Biomedicals, LLC) protein staining assay [21] using KB (nasopharyngeal), HF-6 (colon), MCF-7 (breast), and PC-3 (prostate) cancer cell lines from ATCC, along with a normal skin fibroblast cell line (HFS-30), passage number 33. Cell cultures were maintained in RPMI-1640 medium (Sigma) supplemented with 10% fetal bovine serum (FBS), 5000 units/mL penicillin, 5 mg/mL streptomycin, 7.5% NaHCO3, and cultured in a 96-well microtiter plate (104 cells/mL, 190 μL/well) at 37°C in a 5% CO2-air atmosphere (100% humidity). The cells at the log phase of growth were treated in triplicate with various concentrations of the test extracts (0.16, 0.8, 4, and 20 μg/mL) that were dissolved in RPMI medium supplemented with 0.025% DMSO, and incubated for 72 h in the conditions described above. The cell concentration was determined by the National Cancer Institute (NCI) sulforhodamine method [22]. The optical density was measured at 590 nm with an ELISA-Reader (Molecular Devices, SPECTRA max plus 384). Results were expressed at the concentration that inhibits 50% of control growth after the incubation period (IC50). The values were estimated from a semilog plot of the extract concentration (μg/mL) against the percentage of viable cells. Podophyllotoxin (PTOX) (Sigma) and camptothecin (Sigma) were included as positive standards. Extracts with IC50 ≤ 20 μg/mL were considered active according to the NCI guidelines described in the literature [23].
To determine the specificity of cytotoxic activity, the extracts, were assayed against the normal skin fibroblast cell line (HFS-30), and the selective index (SI) was determined using equation (1):
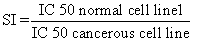
Cell cycle analysis
PC-3 cells (1x105) were plated in 6-well plates and allowed to attach overnight at 37°C in 5% CO2. Exponential growing cells were treated for 72 h with a concentration of the extracts calculated in accordance to IC50 values. Cells from each treatment were trypsinized and collected into single cell suspensions, centrifuged and fixed in cold ethanol (70%) overnight at -20ºC. The cells were then treated with RNase (0.01M) and stained with PI (7.5 µg/mL) for 30 min in the dark, PI has the ability to bind to RNA molecules and hence, RNase enzyme was added in order to allow PI to bind directly to DNA. The percentage of cells in G1, S and G2 phases was analyzed with a flow cytometer (Becton, Dickinson, FACS Calibur, San Jose, CA), the number of cells analyzed for each sample was 10,000. Data obtained from the flow cytometer were analyzed using the FlowJo Software (Tree Star, Inc., Ashland, OR, USA) to generate DNA content frequency histograms, and to quantify the number of cells in the individual cell cycle phases.
Apoptosis analyses
PC-3 cells were seeded in 6-well plates (7.5 x 104 cells/mL) and incubated for 18 h. Exponentially growing cells were treated for 72 h with RPMI medium added with 0.025% of DMSO (control) or in accordance with CI50 of the extracts. Cells treated with H2O2 [24] were used as apoptotic death control, and for the necrotic control PC-3 cells were treated with boiling water. Then each cell culture was washed with 100 mL of PBS and stained with 100 μL AO/EB solution (100 μg/mL-1 AO, 100 μg/mL-1 EB), according to the procedures of Yang et al. [25]. The cells were observed using a fluorescence microscope (Olympus Co, Tokyo, Japan, with emission at 521 nm). AO/EB are intercalating nucleic acid-specific fluorochromes, which emit green and orange fluorescence respectively when they are bound to DNA. It is well known that AO can pass through cell membranes, but EB cannot. Under the fluorescence microscope, living cells appear green. Necrotic cells stain red but have a nuclear morphology resembling that of viable cells. Apoptotic cells appear green, and morphological changes such as cell blebbing and formation of apoptotic bodies are observed. The criteria for identification are as follows: (i) viable cells appear to have green nucleus with intact structure; (ii) early apoptosis cells exhibit a bright-green nucleus showing condensation of chromatin; (iii) late apoptosis appear as dense orange areas of chromatin condensation, and (iv) Orange intact nucleus depict secondary necrosis [13].
Anti-inflammatory activity
In vivo anti-inflammatory experiments were conducted in mice according to the Mexican Guidelines for Animal Welfare NOM-Z00-062-1999 (2013) and were approved by the Ethics and Security Committee from the Centro de Investigación en Biotecnología, UAEM.
Male BALB/c mice weighting between 20 and 25 g were used. Mice were maintained in adequate living conditions at a temperature of 24°C, 70% of humidity with a 12-h light/dark cycle and water/food ad libitum. Animal inflammation was induced using 12-O-tetradecanoylphorbol-13-acetate (TPA from Sigma) by the method previously described by Rao et al. [26]. Under general anesthesia by sodium pentobarbital (3.5 mg/kg, intraperitoneally), 10 µL of TPA in ethanol (0.25 mg/mL) were topically applied on the internal and external surface of the right ear to cause edema. After 10 min of incubation, samples at different doses (from 0.05-1 mg/ear) in ethanol (vehicle) were applied on the same ear. The left ear (negative control) received only 10 µL of ethanol, and 20 µL of vehicle. Indomethacin was used as the anti-inflammatory positive control. After 4 h of incubation with TPA, the animals were sacrificed by cervical dislocation. Circular sections of 7 mm were taken from both ears as samples for further measurement. Increase in the weight of the right ear in respect to the left, was considered edema. The inhibition of edema was calculated by the formula:
% of inhibition=[(C-E) / C] 100
Where C=edema of control group (treated with TPA), and E=edema of experimental group (TPA with compound). Each crude extract was assayed in triplicate as well as the positive control indomethacin. Statistical analysis for anti-inflammatory activity was performed using Prism 6 for Windows version 6.0 (GraphPad Software Inc., La Jolla, CA, USA). Using one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test, p=0.05 was considered to be statistically significant. ED50 values were obtained from regression curves with coefficient factors between R2 =0.80 and 0.98.
The determination of ED50 for the more active extracts was obtained through a curve constructed with four concentrations (0.05, 0.1, 0.5 and 1 mg/ear) and five replicates for each concentration.
Cytotoxicity of extracts
Cytotoxic activities of organic extracts obtained from the nine plant species (eleven populations) are shown in Tables 2 and 3. Results in Table 2 show the cytotoxic values of hexane extracts and reveals that, except for B. glabrifolia (Morelos) and B. excelsa, all the plants exhibited cytotoxic activity (IC50<20 μg/mL) in at least one cancer cell line, with values ranging from 0.39 to 14.26 μg/mL. From the cancer cell lines used to carry out the evaluation, HF-6 and MCF-7 were the most sensitive ones, showing a 72% response for the tested extracts. The least responsive cancer cell line was PC-3, since only four extracts (B. bicolor, B. galeottiana, B. schlechtendalii and B. fagaroides) showed cytotoxic action against this carcinoma. B. linanoe showed a selective cytotoxic effect only toward KB carcinoma (IC50=13.31 μg/mL). Both B. kerberi and B. glabrifolia from Oaxaca, showed cytotoxic action against HF-6 (IC50=6.97 μg/mL), and also toward MCF-7 (IC50=10.29 and 7.82 μg/mL, respectively) being selective against these two cancer cell lines. B. fagaroides was the most active plant against HF-6 (IC50=1.61 μg/mL) and MCF-7 (IC50=2.25 μg/mL) carcinomas and B. schlechtendalii was the one most active against KB (IC5=2.13 μg/mL) and PC-3 (IC50=0.39 μg/mL), although it also exhibited activity against the other two cancer cell lines, as well as toward normal fibroblasts. Even though hexane extracts of B. bicolor, B. lancifolia, B. linanoe, B. kerberi and B. glabrifolia from Oaxaca exhibited toxic effects in at least one cancer cell line, they did not show cytotoxicity against the normal human skin fibroblast cell line HFS-30. On the contrary, B. ariensis from Morelos and México, B. galeottiana, B. schlechtendalii and B. fagaroides showed toxic action against normal fibroblasts.
| Extract | Growth inhibition (IC50, μg/mL)* | ||||
|---|---|---|---|---|---|
| Cell line | |||||
| HF6 | MCF7 | KB | PC3 | HFS-30 | |
| B. ariensis Morelos | 6.34 ± 0.02 | 14.22 ± 0.01 | >20 | >20 | 4.33 ± 0.02 |
| B. bicolor | 7.29 ± 0.05 | 13.76 ± 0.03 | >20 | 9.69 ± 0.01 | >20 |
| B. linanoe | >20 | >20 | 13.31 ± 0.05 | >20 | >20 |
| B. ariensis México | 1.65 ± 0.03 | 2.49 ± 0.05 | 4.38 ± 0.03 | >20 | 1.27 ± 0.03 |
| B. glabrifolia Morelos | >20 | >20 | >20 | >20 | >20 |
| B. kerberi | 6.97 ± 0.03 | 10.29 ± 0.03 | >20 | >20 | >20 |
| B. glabrifolia Oaxaca | 6.96 ± 0.02 | 7.82 ± 0.01 | >20 | >20 | >20 |
| B. galeottiana | 4.67 ± 0.01 | 4.64 ± 0.02 | 14.26 ± 0.02 | 5.94 ± 0.04 | 9.85 ± 0.01 |
| B. fagaroides | 1.61 ± 0.04 | 2.25 ± 0.01 | 6.29 ± 0.03 | 2.13 ± 0.01 | 0.11 ± 0.03 |
| B. excelsa | >20 | >20 | >20 | >20 | >20 |
| B.schlechtendalii | 4.58 ± 0.01 | 9.94 ± 0.02 | 2.13 ± 0.01 | 0.39 ± 0.03 | 7.86 ± 0.01 |
| CTP § | 0.0258 ± 0.02 | 0.0424 ± 0.01 | 0.7855 ± 0.03 | 0.0383 ± 0.02 | 0.5821 ± 0.02 |
| PTOX § | 5.3127E-04 ± 0.01 | 3.7453E-03 ± 0.02 | 4.3833E-04 ± 0.01 | 1.5584E-03 ± 0.01 | 2.82E-02 ± 0.03 |
Table 2: Cytotoxicity of hexane extracts of selected Bursera species from Mexico.
| Extract | Growth inhibition (IC50, μg/mL)* | ||||
|---|---|---|---|---|---|
| Cell line | |||||
| HF6 | MCF7 | KB | PC3 | HFS-30 | |
| B. ariensis Morelos | >20 | >20 | >20 | 6.78 ± 0.04 | >20 |
| B. bicolor | 3.90 ± 0.02 | 11.51 ± 0.03 | >20 | 0.13 ± 0.03 | >20 |
| B. linanoe | >20 | >20 | >20 | >20 | >20 |
| B. ariensis México | >20 | >20 | >20 | 1.87 ± 0.02 | >20 |
| B. glabrifolia Morelos | >20 | >20 | >20 | >20 | >20 |
| B. kerberi | 0.53 ± 0.01 | 1.74 ± 0.03 | 2.93 ± 0.03 | 0.49 ± 0.05 | >20 |
| B. glabrifolia Oaxaca | >20 | >20 | >20 | >20 | >20 |
| B. galeottiana | 3.14 ± 0.04 | 7.25 ± 0.02 | 13.14 ± 0.05 | 2.26 ± 0.04 | >20 |
| B. fagaroides | 1.46 ± 0.01 | 3.71 ± 0.01 | 0.96 ± 0.03 | 1.62 ± 0.02 | 2.74 ± 0.04 |
| B. excelsa | >20 | >20 | >20 | >20 | >20 |
| B. schlechtendalii | >20 | >20 | 6.52 | >20 | >20 |
| CTP § | 0.0258 ± 0.02 | 0.0424 ± 0.03 | 0.7855 ± 0.01 | 0.0383 ± 0.01 | 0.5821 ± 0.01 |
| PTOX § | 5.3127E-04 ± 0.01 | 3.7453E-03 ± 0.02 | 4.3833E-04 ± 0.02 | 1.5584E-03 ± 0.01 | 2.82E-02 ± 0.02 |
Table 3: Cytotoxicity of chloroform extracts of selected Bursera species from Mexico.
Results in Table 3 show the cytotoxic evaluation of the chloroform extracts from the studied species revealing that B. bicolor, B. kerberi, B. galeottiana, B. schlechtendalii, B. fagaroides, and B. ariensis extracts possess cytotoxic activity (IC50<20 μg/mL) with values ranging from 0.13 to 13.14 μg/mL. B. fagaroides showed a generalized cytotoxic action against all carcinomas and also toward normal fibroblasts. B. schlechtendalii showed cytotoxic action only against KB (IC50=6.52 μg/mL) and no action against normal fibroblasts. B. bicolor was the most active one against PC-3 (IC50=0.13 μg/mL), and presented an outstanding SI (Selective Index) (SI=153.85), also showing cytotoxicity toward the other two carcinomas (HF-6 and MCF-7), but with no action against normal fibroblasts. Similarly B. kerberi and B. galeottiana extracts were active against the four carcinomas, with no effects against normal fibroblasts; B. kerberi presented an important SI for HF-6 and PC-3 cell lines (37.74 and 40.82, respectively). For the ethyl acetate extracts, results of the cytotoxic evaluation revealed that only B. fagaroides was active, and this activity was exerted against the four carcinomas HF-6 (IC50=3.46), MCF-7 (IC50=4.06 ), KB (IC50=2.67 ) and PC-3 (IC50=4.04 μg/mL). Notably, the methanolic extracts obtained from all the studied species did not exhibit cytotoxicity in any of the studied carcinoma cell lines (data not shown).
Cell cycle arrest
To assess the growth inhibitory effect on normal cell cycle progression on the basis of cytotoxic assay results and availability of samples, we conducted a cell cycle analysis measuring intracellular DNA content through flow cytometry in B. ariensis from Mexico and Morelos, B. galeottiana and B. kerberi. The status of the cell cycle of PC-3 cells treated with the four chloroform extracts for 72 h was analyzed, and PTOX was included as a positive control. As shown in Figure 1, exposure of PC-3 cells to chloroform extracts caused the appearance of a population in the sub-G1 region of the profile where apoptotic cells are found. Importantly, the treatment of PC-3 cells with extracts of B. galeottiana (Figure 1e) increased the G2/M populations, and correspondingly decreased G0/G1 phase in relation to the negative control (Figure 1a); whereas the treatment with B. ariensis from Morelos and from the state of Mexico (Figures 1c and 1d) presented similar apoptotic cells in the sub G1 phase to those of the positive control PTOX (Figure 1b). Only B. kerberi did not show a clear change in any phase of the cell cycle (Figure 1f).The above results necessitated performing apoptosis assay
Apoptosis assay by AO/EB staining method
The cytotoxic assay and cell cycle analyses indicated apoptosis when using the chloroform extracts from B. ariensis from Mexico and Morelos, B. galeottiana and B. kerberi. These results were confirmed by fluorescence microscopy analysis. The cells were scored under the fluorescence microscope in order to quantify viable cells. The analysis was repeated in triplicate and the images were captured in fluorescence and phase contrast at the same field, and in 10 and 40 x magnification. Fluorescence microscopic analysis showed that the untreated PC-3 cells were stained with uniform green fluorescence (Figures 2a and 2c); PC-3 cells in early apoptosis were stained with bright spots in green fluorescence (Figures 2b PTOX; 2e B. ariensis from Morelos; 2f B ariensis from the state of Mexico; 2g B. galeottiana and 2h B. kerberi.), in late apoptosis PC-3 cells were stained in orange fluorescence with condensed and fragmented cell nucleus (Figures 2g B. galeottiana and 2h B. kerberi), and in necrosis PC3 cells were stained in orange fluorescence, but nucleus morphology appeared without condensation of chromatin (Figure 2d, H2O2).
Figure 2: Phase and fluorescent micrographs of acridine orange and propidium iodide double-stained PC-3 cells. Cells were treated at IC50 values of extracts from B. ariensis from the state of Mexico and Morelos (e and f respectively), B. galeottiana (g) and B. kerberi (h) in a time-dependent manner. Cells were cultured in RPMI 1640 media maintained at 37°C and 5% CO2. Untreated cells after 72 h showed normal structures without prominent apoptosis and necrosis (a). Blebbing and orange color representing the hallmark of late apoptosis were noticed by g and h, early apoptosis features were represented by intercalated acridine orange (bright green) amongst the fragmented DNA (b, c and e-h), and red bright colored necrosis was observed by H2O2 treatment (d). Images are representatives of one of three similar experiments. Fluorescence and phase contrast images were captured at the field. Fluorescence images of samples were captured at 10x and 40x magnification.
Anti-inflammatory assay on ear edema induced by TPA in mice
The anti-inflammatory effects of the burseras extracts are shown in Figure 3, and expressed as percentage of edema inhibition (% EI). The data indicated an important loss of weight by the extracts of B. galeottiana (89.92%), B. excelsa (90.79%) and B. schelechtendalii (91.60%), The Dunnett´s test indicated that these three species were the most active extracts and statistically comparable to the control indomethacin. The best extracts with anti-inflammatory potential were selected to determine the average effective dose (ED50). B. galeottiana was the most active extract (ED50=0.23 ± 0.02 mg/ear) when compared to the positive control indomethacin (ED50=0.19 ± 0.02 mg /ear) (Table 4).
| Sample | CI50 (mg/mL) |
|---|---|
| B. excelsa | 0.26 ± 0.01 |
| B. galeottiana | 0.23 ± 0.02 |
| B. schlechtendalii | 0.25 ± 0.02 |
| Indomethacine | 0.19 ± 0.02 |
Table 4: Anti-inflammatory activity of chloroform extracts from selected burseras.
Figure 3: Anti-inflammatory activities of selected species of the Bursera genus. 1. Indomethacin, 2. B. ariensis from Morelos, 3 B. bicolor, 4. B. linanoe , 5. B. ariensis from Mexico state, 6. B. glabrifolia from Morelos, 7. B. kerberi , 8. B. glabrifolia from Oaxaca, 9. B. galeottiana , 10. B. fagaroides , 11. B. excelsa, 12. B. schlechtendalii.
Plants have a long history of application in the treatment of cancer, but many of the claims for the efficacy of such treatment should be viewed with some skepticism because cancer, as a specific disease entity, is likely to be poorly defined in terms of folklore and traditional medicine [27]. In our case, besides the claimed popular use of these plants, the chemotaxonomic approach proved to be effective, since it was possible to identify Bursera species with cytotoxic activity indicating or affirming its usefulness as a method of selection.
With the exception of B. glabrifolia from Morelos and B. excelsa, all the studied bursera species exhibited cytotoxic activity. B. fagaroides proved to be the most active plant studied in this investigation since all three extracts (hexane, chloroform and ethyl acetate) displayed important cytotoxic effect against the four carcinoma cell lines HF-6, MCF-7, KB and PC-3. The obtained results are in accordance with those previously reported by other authors who studied this plant but from a population collected in the state of Michoacán [11,16]. B. schlechtendalii showed important cytotoxic activity, and this finding is in accordance with McDonield and Cole [18] study, since they identified two lignans with anti tumor activity in this species. In the case of B. ariensis notable cytotoxic effects from the hexane extract against all the cancer cell lines were obtained, and although there are no previous reports that evaluate the cytotoxic activity of this species, a lignan, denominated ‘ariensin’, was isolated from B. ariensis growing in the state of Michoacán [27,28], and was then synthesized by Burke and Stevenson [29], as well as later identified in Acanthopanax koreanum [30].
Koulman in 2003 [31] did not perform cytotoxic studies in B. excelsa collected in the state of Mexico but he did isolate three compounds: iso-bursehernin,3,4-dimethoxy-3’,4’-methylenedioxylignano-9,9’-epoxylignan-9’-ol and guayadequiol, that he proposed as substrates for the synthesis of cytotoxic lignans. Some recent studies using NMR spectra of B. ariensis and B. fagaroides conducted by our research group indicated the presence of characteristic signals of aryltetralin lignans, whose structures are oxidized at C9 and C9’ (signals situated at 4.56 ppm in the 1H NMR spectra). Since lignans are important compounds with attributed cytotoxic activity, the isolation and identification of these metabolites from the two mentioned species is an important accomplishment.
In our study, the hexane extract of B. kerberi showed cytotoxic effect against HF-6, MCF-7 and, the chloroform extract showed cytotoxic effect against HF-6, MCF-7, KB and PC-3. Hernandez et al. in 2005 [32] reported the isolation of verticillane derivates from B. kerberi collected in Jalisco; and although there are no cytotoxic data from these diterpenes, they were proposed as biogenetic precursors of the cytotoxic taxanes. Several reports in the literature do show cytotoxic activity displayed by triterpene compounds obtained from different species such as Glochidion eriocarpum, Bridelia cambodiana and species of Protium genus [33-35]. Some phytochemical studies report the presence of triterpenes in the Bursera genus [36-39], but only one investigation of the anti-tumoral activity of two triterpenes known as ‘sapeline’ A and B, isolated from B. klugii, showed activity against the P-388 lymphocytic leukemia test system (3PS), as well as toward the human epidermoid carcinoma of the nasopharynx (KB) [19]. In ongoing investigations conducted by our research group on the Bursera species selected for this study; B. bicolor, B. lancifolia, B. linanoe, B. kerberi, B. excelsa, B. galeottiana and B.glabrifolia showed 1H NMR spectra with characteristic triterpene signals profiling from 0.5 to 2.5 ppm . Extracts from these species also gave a positive Liebermann-Buchard test, which is characteristic of triterpenoids.
The lack of cytotoxic activity from B. bicolor, B. kerberi and B. galeottiana extracts on normal fibroblasts HFS-30 merits further studies in search of potential remedies with therapeutic value, since they exert high cytotoxic activity against cancer cell lines. B. bicolor and B. kerberi chloroform extracts showed outstanding selective cytotoxicity against PC-3 cancer cell line (SI=153.85 and 40.82, respectively), and only B. kerberi showed important selective cytotoxicity against HF-6 (SI=37.74).
It is important to note that while B. glabrifolia collected in Oaxaca was toxic against HF-6 and MCF-7 cell lines, the population of this species collected in Morelos was non-toxic against the tested cancer cell lines. This variation indicates different chemical profiles between both populations, possibly related to geographical, climatic or soil conditions.
In relation to cell cycle analysis of their the DNA content, cells can be classified into three categories: cells in G1 do not present DNA duplication, cells in G2/M exhibit duplicated DNA, and cells in S phase present intermediate DNA content. In each phase of the cell cycle, important control mechanisms exists that involve checkpoints which ensure the proper execution of cell cycle events. The p53 protein checkpoint blocks the entry of cells to mitosis when DNA is damaged in G2/M phase. This protein (p53) can activate the transcription of several apoptosis associated genes to program cell death in response to genotoxic stresses [40,41]. In our study, the results show that with exception of B. kerberi; the chloroform extracts can effectively induce apoptosis in PC-3 cells. These findings support those results obtained with cell cycle and cytotoxic experiments. B. galeottiana chloroform extract showed an important inhibitory effect related to G2/M phase arrest, and it remains to be demonstrated if this is due to the inhibition of the p53 checkpoint. At this phase, cells undergo apoptosis since they cannot be repaired
The observation of apoptosis induced by the plant extracts could be a key factor in determining their efficacy, in as much as most tumor cells remain sensitive to some apoptotic stimuli from anticancer drugs.
An inflammation-cancer relation has been proposed many years ago, and the interaction between them has become more and more evident. Inflammation is an immediate host defense mechanism of the body to tissue injury caused by noxious stimuli, and is characterized by the release of mediators (prostaglandins, cytokines, chemotactic molecules and vasoactive peptides), which affect the cellular infiltration and vascular permeability. For example, colorectal cancer is linked to colitis, which increases the risk of colorectal cancer by 10-fold, and the treatment of patients with anti-inflammatory therapy reduces this risk [42]. The role of inflammation in epigenetic, and changing genetic events associated with cancer is estimated in more than 25% of all cancers [43].
Two of the studied species (B. galeottiana, and B. schlechtendalii) showed important anti-inflammatory activity comparable to the control indomethacin, as well as cytotoxic effects against cancer cell lines. As mentioned in the introduction, several reports in the Bursera genus had identified triterpenes as the involved compounds the in anti-inflammatory response. The triterpenoids of the oleanane and ursane series displayed anti-inflammatory activity [44] and were isolated from B. simaruba leaves, B. graveolens and B. lancifolia [44-46].
The overall results of this investigation postulate the studied species of the Bursera genus as candidates to investigate for their therapeutic potential in the treatment of cancer diseases and inflammation. Clearly, our results are an important contribution to the understanding on the potentialities of the Bursera genus as a source of new medical approaches.
This is the first report that focuses a systematic study on the cytotoxic and anti-inflammatory activities of nine species (eleven populations) of the Bursera genus growing in Mexico. The correlation of this information with ethnomedical data will be of great benefit for the rational use of these plant species.