Journal of Horticulture
Open Access
ISSN: 2376-0354
ISSN: 2376-0354
Research Article - (2017) Volume 4, Issue 3
Tillandsia recurvata has anticancer properties as well as horticultural value. Production through seed germination is non-viable since the species produce low numbers of seeds with low viability, longevity and germination rates. We explored propagation using cuttings and tested different inert substrates and plant growth, regulators. In 2008, cuttings were taken from T. recurvata plants and sown in/on three different substrates (red lava rock, gravel and electricity cable) to which growth regulating cytokinins [6-Benzilaminopurine (BAP) and Kinetin (KIN)] were added. The effects of substrate and cytokinin addition were determined in terms of cutting survival and four different relative growth indices (length, weight, number of live leaves and the number of new leaves). Cutting weight, a number of new leaves and survival did not differ among the different substrate types and plant growth regulators. The cuttings sown on cable presented a higher quantity of live leaves. The cutting length was greater in the control or with the addition of KIN; the length was also greater when the cuttings were sown in red lava rock. The red lava rock and cable are both substrates with potential for propagation of T. recurvata; however, given the lack of differences found between the control and KIN treatments, the addition of these plant growth regulators appears to be unnecessary. We conclude that sowing cuttings in/on inert substrates constitute an inexpensive and easily implemented technique for propagation of this species.
<Keywords: Epiphytes; Tillandsia recurvate; Propagation; Plant growth regulators
Many wild plant species are extracted from their environment because they are of ornamental value [1] and/or represent a source of chemical compounds (i.e., natural products with therapeutic properties) [2], which give them a measure of ethnobotanical value [3]. However, chronic extraction of plants can negatively impact populations and have repercussions at both community and ecosystem levels [4]. As a consequence, efforts have been made towards the generation of biotechnological [5] and/or agricultural (e.g. domestication [6]) protocols that permit the sustainable management of populations under extraction. The search for adequate substrates for plant propagation is also important [7], as is examination of the effects of plant growth regulators [8]. These factors can act to optimize the survival and growth of artificially propagated plants.
Members of the family Bromeliaceae have different ethnobotanical uses [9]; species of the genus Tillandsia are often used in religious festivals [1,10,11] and are also useful from a horticultural perspective [12], while others have therapeutic properties [9,13,14]. The epiphyte Tillandsia recurvata (L.) has multiple uses, since extracts of this species are cytotoxic to some human carcinogenic cellular lines [15], in addition to the allelopathic [16] and horticultural [12] potential of some individuals.
T. recurvata is an important species, since it reincorporates nutrients to the ecosystem [17], interacts with its phorophytes [18] and other epiphytes [16] and participates in multi specific interactions (xylophage-host-epiphyte [19,20]. It is an atmospheric species (i.e., the roots are for anchorage only and nutrients are acquired directly from the environment) [21] of wide distribution and is found from the southern United States to Argentina [22]. It can complete its life cycle on inert substrates such as electricity cables [23] and is highly abundant in some forests [24,25]. However, its fruits contain few seeds (average 50 seeds) [12,26,27] of approximately 2.5 mm in length with a white plumose appendage [22], which present lower longevity and germination than other sympatric Tillandsia species (e.g. Tillandsia caput-medusae) [12]. One alternative to propagation by seed germination is the development of propagation technologies that generate information about the factors that act to promote the growth of Tillandsia recurvata and allow the generation of individuals with similar characteristics suitable for use as ornamental plants and/or sources of natural products. Apart from one study [28] conducted in wild conditions, there is a lack of information about the growth of atmospheric Tillandsia species. In this study, we suggest the use of cuttings taken from T. recurvata individuals as a direct and rapid method of propagation. The objective of this study was to evaluate the growth and survival of T. recurvata cuttings in different inert substrates and assess the effect of adding different plant growth regulators. We hypothesized that, as an atmospheric species, cuttings of T. recurvata would present greater survival and growth in substrates where they are more exposed and that the addition of plant growth regulators would act to increase their survival and growth.
Collection site and study species
In January 2008, 35 Tillandsia recurvata individuals were collected in San Andrés de la Cal, Tepoztlán, Morelos, Mexico (8º57ˈ22.2ˈˈ W; 99º06ˈ50.2ˈˈ N, 1495 m a.s.l.). The dominant vegetation type at the collection site is tropical deciduous forest (For more information about the collection) [12,20,24].
The epiphytic bromeliads found at the study site include Tillandsia achyrostachys E. Morren ex Baker, Tillandsia caput-medusae E. Morren, Tillandsia hubertiana Matuda, Tillandsia schiedeana Steud., Tillandsia ionantha Planch., Tillandsia makoyana Baker, Tillandsia recurvata and Viridantha atroviridipetala (Matuda) Espejo. T. recurvata accounts for 72% of the epiphytes present [24]. Plants of this species are herbaceous epiphytes, rupicolous or growing on suspended cables, acaulescent, the leaves have a dense covering of trichomes, which gives them a whitegrey color, and they are verticillate, forming a tussock of leaves. A tussock is considered to be a group of T. recurvata plants that form a spherical (5-10 cm diameter) and discrete cluster, where all of the leaves come from the center of the sphere and delimit the tussock with their tips [29]. In each tussock, there are different branches with a few leaves distributed in a distichous form.
Experimental design
The collected plant material was transported to the Biotechnology Laboratory of the Centro de Desarrollo de Productos Bióticos-Instituto Politécnico Nacional (CeProBi-IPN) and 7-9 cuttings of similar size and shape were obtained from each plant. A cutting is a module of the plant (e.g. branches) separated from its progenitor that can give rise to an independent individual (clone) with genetic material identical to that of the plant from which it was originally obtained [30]. In this study, a T. recurvata branch was considered a cutting. Prior to sowing, the identity of each cutting was recorded by labeling them with progressive numbers (1 to 270) and the length (mm) and weight (g) of each was recorded, along with the number of leaves.
The cuttings were sown in flat polystyrene germination trays (28.5 × 55 cm × 6 cm). The trays contained one of the three different substrates (red lava rock, gravel and electricity cable). The red lava rock (trademark PISUMMA, size 1.5 cm) and gravel (Trademark PISUMMA, white color, size small) were poured into the trays until reaching a uniform depth of 4 cm. To install the cable (Model: Indiana 8.37 mm2, 8-AWG), two perforations were made at each end of the trays and the cable was attached between each pair of perforations, providing two fixed cables per tray. Ten randomly-assigned cuttings of T. recurvata were sown in each tray. In the case of the trays with cables, five cuttings were attached to each cable equidistantly using covered twist-tie wires. The trays were placed in a greenhouse and the position of each tray was assigned randomly. The relative humidity and temperature of the greenhouse was monitored hourly (Onset HoBo ProSeries, model H08-32-IS) over the course of the experiment (January-March of 2008). The monthly average greenhouse temperatures during the experiment were: January (mean ± SD) 20.91ºC ± 0.62ºC, February 21.41ºC ± 8.97ºC and March 22.54 ± 9.14ºC. Relative humidity was 42.78% ± 21.75% in January, 34.38% ± 17.26% in February and 33.05% ± 0.99% in March.
According to the treatment in each tray, the plants were sprayed with the cytokinins 6-Benzylaminopurine [(BAP) (0.2 mg/L, Sigma B-3408)] or 6-Furfurylaminopurina [(KIN) (0.2 mg/L, Sigma K-0753)] or with distilled water containing no growth regulator (control). These plant growth regulators were chosen because they promote cellular division and break the dormancy of lateral buds [31]. The cytokinin concentrations were low since the plants were being sprayed every third day. At the end of the experiment, the cuttings were removed from the trays and their length and weight recorded, along with the number of new leaves, live leaves and the number of cuttings that remained alive. Based on the measurements of the cuttings before and after sowing, a relative growth index was obtained for each of the variables, using the following formula:
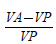
Where VA is equal to the value of the variable (e.g. weight) after sowing the cutting and VP is the value of the variable prior to sowing. A positive value of the index indicates growth.
Statistical analysis was conducted using an ANOVA with two factors [32], where one factor was the “growth regulator” with three levels: BAP, KIN and No Regulator (NR), and the other were “substrate type”, also with three levels: red lava rock, gravel and cable. The combinations of the levels of each factor gave a total of nine treatments (BAP-red lava rock, BAP-gravel, BAP-cable, KIN-red lava rock, KINgravel, KIN-cable, NR-red lava rock, NR-gravel, NR-cable). Within each treatment, there were three trays or units (N=27) with 10 cuttings in each tray (N=270). The response variables were the relative growth indices of cutting length, weight, new leaves, live leaves and survival measured as the number of live cuttings remaining in each tray. All analysis was conducted using the program Stata 13 [33].
Prior to sowing, the length of the cuttings of Tillandsia recurvata was 48.84 mm ± 14.77 mm, while the weight was 0.31 g ± 0.13 g and the number of leaves was 8.67 ± 2.27. Relative growth rate in weight of the cuttings did not differ among the different substrate types (F2,18=0.31, P=0.73; cable=0.08 g ± 0.96 g, gravel=-0.03 g ± 0.09 g, red lava rock=- 0.12 g ± 0.04 g), neither the growth regulator (F2,18=0.85, P=0.44; BAP=-0.14 g ± 0.08 g, KIN=-0.10 g ± 0.06 g, NR=0.17 g ± 0.93 g) nor the interaction between substrate and regulator (F2,18=0.69, P=0.60; Table 1) affected this variable.
| Substrate | Plant Growth Regulator | Survival (Proportion of alive cuttings) |
Relative Growth Rate (Mean ± SE) | ||
|---|---|---|---|---|---|
| Weight (g) | Number of new leaves | Number of live leaves | |||
| Red lava rock | KIN | 0.97 ± 0.58 | -0.12 ± 0.02 | 0.38 ± 0.14 | 0.17 ± 0.09 |
| BAP | 0.97 ± 0.58 | -0.11 ± 0.03 | 0.31± 0.10 | 0.21 ± 0.08 | |
| Control | 0.97 ± 0.58 | -0.14 ± 0.07 | 0.54 ± 0.19 | 0.27 ± 0.14 | |
| Gravel | KIN | 0.93 ± 0.11 | -0.05 ± 0.03 | 0.24± 0.22 | 0.14 ± 0.10 |
| BAP | 0.93 ± 0.05 | -0.08 ± 0.08 | 0.14± 0.07 | 0.20 ± 0.20 | |
| Control | 0.93 ± 0.11 | 0.02 ± 0.14 | 0.86 ± 0.81 | 0.23 ± 0.19 | |
| Cable | KIN | 0.96 ± 0.05 | -0.15 ± 0.07 | 0.13 ± 0.15 | 0.41 ± 0.27 |
| BAP | 1.00 ±0.00 | -0.24 ± 0.05 | 0.33 ± 0.06 | 0.47 ± 0.10 | |
Table 1: Mean ± SE of the variables measured in cuttings of Tillandsia recurvata. Values correspond to the interaction of substrate × plant growth regulator.
Survival of the cuttings (measured as live cuttings per tray) was also unaffected by substrate type (F2,18=0.92, P=0.0.41; cable=0.97 ± 0.04, gravel=0.93 ± 0.08, red lava rock=0.96 ± 0.05), growth regulator (F2,18=0.07, P=0.93; BAP=0.96 ± 0.05, KIN=0.95 ± 0.07, NR=0.95 ± 0.07) or by the interaction between substrate and regulator (F2,18=0.07, P=0.98; Table 1).
Relative growth rate in the new leaves did not differ among the three different substrates (F2,18=0.18, P=0.82; cable=0.63 ± 1.39, gravel=0.41 ± 0.64, red lava rock=0.41 ± 0.16), with the addition of the different plant growth regulators (F2,18=1.82, P=0.19; BAP=0.26 ± 0.11, KIN=0.25 ± 0.18, NR=0.94 ± 1.37) or with the interaction between substrate and regulator (F2,18=0.35, P=0.83; Table 1).
Substrate type did influence the relative growth rate in live leaves per cutting (F2,18=3.90, P=0.03), where those sown on the cable (0.39 ± 0.18) presented a higher quantity of live leaves than those sown in gravel (0.19 ± 0.15), while the relative growth rate of those sown in red lava rock was intermediate (0.21 ± 0.10, Tukey P<0.05). Neither growth regulator (F2,18=0.24, P=0.78; BAP=0.29 ± 0.17, KIN=0.24 ± 0.19, NR=0.26 ± 0.16) nor the interaction between growth regulator and substrate type affected the growth of the live leaves (F2,18=0.50, P=0.72; Table 1).
Substrate type also affected the growth rate in terms of cutting length (F2,18=7.21, P=0.005); cuttings sown in the red lava rock were longer (0.10 mm ± 0.06 mm) than those sown on cable (0.005 mm ± 0.12 mm), while those sown in gravel actually lost length (-0.002 mm ± 0.04). The addition of growth regulators also affected the final length of the cuttings (F2,18=4.02, P=0.03); cuttings with KIN (0.69 mm ± 0.08 mm) were longer than those with BAP (-0.01 mm ± 0.08 mm), while the control did not differ from either cytokinin treatment (0.05 mm ± 0.09 mm). Growth regulator and substrate type interacted marginally in terms of cutting length (F2,18=3.12, P=0.04; Figure 1). The longest cuttings were those sown in red lava rock with no growth regulator (0.69 mm ± 0.08 mm), those sown in red lava rock with added KIN (0.09 mm ± 0.06 mm) and those sown on cable with added KIN (0.12 mm ± 0.09 mm, Figure 1). Cuttings sown on cable with added BAP presented a negative index of length (-0.09 mm ± 0.08 mm; Figure 1).
Figure 1: Length of Tillandsia recurvata cuttings sown in three different substrates with addition of two different plant growth regulators and a control with no added regulator (■=BAP, ●=KIN, ♦=control). Different letters indicate significant differences (Tukey test, P<0.05). Points represent mean values and bars represent 95% confidence intervals.
Tillandsia recurvata presents a CAM metabolism [34] that is adapted to stressful conditions. Among other attributes (anemochory, autogamy) [35], this metabolism type confers advantages in terms of colonization of the canopy in arid and semi-arid environments [24,25]. As with other Tillandsia species [36], T. recurvata can establish itself and grow abundantly on inert substrates, such as suspended cables [23]. In this study, we found that the cuttings of T. recurvata transplanted onto cable presented a higher number of live leaves than those sown in red lava rock, but an equal number of leaves as those presented by the cuttings sown in gravel. This may be due to the atmospheric habit of this species [21]; i.e., growing on suspended cable implies that the plant is more exposed in terms of water and nutrient capture, while excess water is eliminated to the atmosphere. The water that T. recurvata acquires through its leaves enters its tissues, but if excess water is not eliminated, this can interfere with respiration, increasing leaf mortality. On the cable, the cuttings can generate leaves without increasing stem length, but in the two other inert substrate types there were negative costs to the leaves and increases in stem length. Unlike the cable, red lava rock is a porous substrate that can store water in its cavities [7], while the gravel does not accumulate water in its pores, but only between its conglomerate units. It is possible that higher leaf mortality occurs as a result of not eliminating moisture, generating fewer live leaves and causing lower growth of the plant in these substrates. The cuttings in red lava rock had as many live leaves as those on the cable and presented increased length. This growth pattern could be a strategy adopted by the cuttings in order to escape the effects of excess moisture.
There is evidence that when plants of T. juncea are small they grow faster [28]. When our experiment began, the cuttings of T. recurvata measured 48.84 mm of height, which is similar to the height of T. brachycaulus seedlings (<0.4 cm) or infants (0.5-2.4 cm) [37]. Accordingly, we expected that three months would be enough to detect a conspicuous growth in cuttings of T. recurvata; however, growth in T. recurvata cutting length was lower than in other atmospheric Tillandsia species. It has been reported that T. butzii and T. juncea have an annual growth rate of 2.8 cm ± 0.28 cm and 3.5 cm ± 0.28 cm respectively [28]. Without considering the effects of environmental conditions throughout the year, this implies that monthly average growth would be 0.23 cm and 0.29 cm for T. butzii and for T. juncea, respectively. These values are higher than the maximum growth rate we found for T. recurvata (0.15 mm equivalent to 0.015 cm). It is possible that this low growth rate of T. recurvata is a result of the stressful conditions of dry tropical forest, while T. butzii and T. juncea inhabit lower montane cloud forests, where water is not a limiting factor.
The cytokinins BAP and KIN are frequently used in biotechnological protocols since they induce the development of buds and increase leaf growth due to foliar expansion as a consequence of cellular division and lengthening [38]. In this study, we found that the cuttings to which KIN were added were longer at the end of the experiment than those to which BAP were added. However, addition of KIN to the cuttings did not produce results that differed significantly from those of the control. This suggests that, in this case, addition of plant growth regulators is probably unnecessary because addition of these cytokinins by irrigation means that they are absorbed via the leaf stomata, a common procedure among atmospheric epiphytes, while when they are administered to explants cultivated in vitro, they are absorbed by the roots.
T. recurvata is a very abundant species in some zones [24-26]; however, there is evidence that the abundance of such species of horticultural and ethnobotanical value has been impacted by human extraction [1]. For this reason, it is important to consider alternatives for propagation within conservation plans that select the most desired phenotypic characteristics of the T. recurvata plants.
The results of this study demonstrate that red lava rock was the substrate in which the plants maintained the highest number of live leaves and presented greatest growth. With respect to the addition of plant growth regulators, at the end of the experiment, the cutting length was equal with or without the addition of KIN. It can therefore be concluded that the choice of substrate for sowing cuttings of Tillandsia recurvata is a more important factor than the addition of plant growth regulators. It is therefore suggested to use red lava rock for the purposes of propagation of plants of this species. Finally, given that the final length of the cuttings did not differ between the KIN and control treatments, the use of plant growth regulators appears to be unnecessary at least in the case of BAP and KIN.
We are grateful to T. Cruz-Fernández, A. Martínez-Ray, and S.E. Flores- Valencia for their help with work in the greenhouse. We appreciate the comments of K. Macmillan to the ms. This study formed part of the Doctorate studies of SVD. We thank the Consejo Nacional de Ciencia y Tecnología for the scholarship (87232) awarded to SVD.