Rheumatology: Current Research
Open Access
ISSN: 2161-1149 (Printed)
ISSN: 2161-1149 (Printed)
Research Article - (2023)Volume 13, Issue 5
Objective: Step Therapy (ST) policies are implemented by third-party payers to reduce prescription drug costs in Rheumatoid Arthritis (RA), often resulting in payer preference for a Tumour Necrosis Factor inhibitor (TNFi) after patients fail conventional synthetic Disease-Modifying Antirheumatic Drugs (csDMARD) therapy. Commercial availability of PrismRA has provided rheumatologists with a clinically validated, personalized medicine approach for identifying RA patients who might benefit from first-line use of a non-TNFi biologic/targeted synthetic DMARD (b/tsDMARD) after failing csDMARD therapy. We sought to determine the extent to which ST policies impede RA patients’ access to non-TNFi b/tsDMARD therapy when their PrismRA indicated high likelihood of inadequate response to TNFi therapy.
Methods: We performed chart review of all RA patients in our practice whose PrismRA indicated high likelihood of inadequate response to TNFi therapy. We collected data on patients’ previous history of b/tsDMARD exposure, payer identity, and payer approval/rejection determination of the non-TNFi b/tsDMARD prescribed following the PrismRA result.
Results: 150 RA patients had a PrismRA test performed, of which 41 patients had a high likelihood of inadequate response to TNFi therapy and were subsequently prescribed a non-TNFi b/tsDMARD. 71% of patients had their non-TNFi b/tsDMARD approved by their payer without need for appeal. Approval was higher for patients with Medicare coverage (85%) compared commercial insurance (64%), though was similar for patients who were TNFi naïve (76%) and TNFi exposed (65%).
Conclusion: A majority (71%) of RA patients in our practice with a PrismRA indicating high likelihood of inadequate response to TNFi therapy were able to successfully access their non- TNFi b/tsDMARD prescribed in alignment with their PrismRA result.
Step therapy; Rheumatoid arthritis; Personalized medicine; Managed care; Disease-modifying antirheumatic drugs
Step Therapy (ST), also known as “Step Edit” or “Fail First”, is a policy adopted by third-party payers to minimize costs of prescription drug use by requiring patients to trial a less expensive, first-line medication before second-or third-line options are reimbursed by a patient’s health plan [1]. While the reduction of healthcare spending intended by ST protocols is a worthy aim, studies have shown that ST policies may harm patient outcomes by limiting prescriber options and necessitating proof-of-failure of less effective or less well-tolerated medications [2,3]. Given the high costs of biologic and targeted synthetic DMARDs (b/tsDMARDs) for the treatment of Rheumatoid Arthritis (RA), ST has had wide implementation by third-party payers for reducing costs of RA care, usually resulting in a first-line preference by the patient’s health plan for a Tumour Necrosis Factor inhibitor (TNFi) after the patient has failed conventional synthetic Disease-Modifying Antirheumatic Drugs (csDMARD) therapy [4]. As a result, up to 90% of RA patients are treated with a TNFi as their first-line b/tsDMARD despite the fact that current American College of Rheumatology (ACR) practice guidelines do not endorse TNFi therapy over other b/tsDMARD classes for such patients [5-7]. Unfortunately, many RA patients do not meaningfully improve with use of TNFi therapy, which may lead to poorer quality of life as well as increased joint damage, disease- related morbidity and healthcare resource utilization for these patients [8-10].
Until recently, rheumatologists lacked clinically validated markers to guide b/tsDMARD selection for individual RA patients, and therefore reasons to bypass use of TNFi therapy as a patient’s first-line b/tsDMARD were rare. In 2020, however, a Molecular Signature Response Classifier (MSRC) test known as PrismRA® became commercially available for predicting an individual RA patient’s likelihood of inadequately responding to TNFi therapy [11-15]. PrismRA uses 23 biologic features; including 19 gene transcripts, 1 serologic marker and 3 clinical features, which are unique to each RA patient to generate a prediction of inadequate response to TNFi, defined as failure to achieve an ACR50 improvement with 24 weeks of TNFi therapy. PrismRA prediction results are divided into 3 categories: 1) high TNFi inadequate response signature detected, indicating the patient has a 90% likelihood of inadequate response; 2) very high TNFi inadequate response detected, indicating the patient has a 95% likelihood of inadequate response; or 3) no TNFi inadequate response detected. In the NETWORK-004 clinical validation study of PrismRA, b/ tsDMARD-naïve RA patients with a PrismRA result indicating a high or very high likelihood of inadequate response to TNFi therapy and then treated with a TNFi medication were 3.6 and 8.8 times less likely to achieve Clinical Disease Activity Index (CDAI) low disease activity (CDAI ≤ 10) and CDAI remission (CDAI ≤ 2.8), respectively, compared to RA patients treated with a TNFi therapy who had a PrismRA result that lacked an inadequate response signal [11]. The release of PrismRA has thus equipped rheumatologists with a personalized, clinically validated biomarker test to identify the subset of RA patients within their practice who might benefit from first-line use of a non-TNFi b/tsDMARD after failing csDMARD therapy. A perceived obstacle to implementing results from the PrismRA test in clinical practice, however, is ST protocols that may mandate first-line use of a TNFi therapy. The ACR has published a position statement asserting that ST programs should not interfere with access to medically appropriate DMARD therapies for individual RA patients [16].
To determine the extent to which ST policies impede a RA patient’s access to non-TNFi b/tsDMARD therapy when their PrismRA test result predicted likely inadequate response to TNFi therapy, we set out to analyze our experience in a real-world community rheumatology practice. We hypothesized that ST protocols would prohibit a majority of patients from using a non-TNFi b/tsDMARD first-line even when their PrismRA test result indicated a high or very high likelihood of TNFi inadequate response.
To determine the extent to which ST policies impede a RA patient’s access to non-TNFi b/tsDMARD therapy when their PrismRA test result predicted likely inadequate response to TNFi therapy, we set out to analyze our experience in a real-world community rheumatology practice. We hypothesized that ST protocols would prohibit a majority of patients from using a non-TNFi b/tsDMARD first-line even when their PrismRA test result indicated a high or very high likelihood of TNFi inadequate response.
We performed a retrospective chart review of all RA patients in our practice who had a PrismRA test performed in 2021-2022 with a result indicating a high (>90%) or very high (>95%) likelihood of inadequate response to TNFi therapy. We collected data on each patient’s previous history of b/tsDMARD use, b/tsDMARD prescription that was guided by the PrismRA result, third-party payer status, and payer approval/rejection determination related to the b/tsDMARD prescription placed by their rheumatologist following the PrismRA result. Patients who had a PrismRA result but had not yet had a b/tsDMARD prescribed following the result or whose b/tsDMARD prescription did not align with the PrismRA result were excluded from the analysis. Patients who had their initial PrismRA-guided b/tsDMARD prescription denied by their third-party payer but were offered an in-class alternative with a similar mechanism of action (i.e. sarilumab in place of tocilizumab; upadicitinib in place of tofacitinib, etc.) were considered as being approved for purposes of our analysis. Given that Medicare makes prescription coverage determinations based upon medical necessity, which can often still leave patients with unaffordable copays, we also collected data on Medicare patients regarding if they received and started their PrismRA-guided b/tsDMARD therapy. Patients with an Original Medicare policy with Part B or D coverage or with a Medicare Advantage plan who were unable to receive and start their prescribed PrismRA-guided b/tsDMARD due to an unaffordable copay were considered as denied for purposes of our analysis. In some cases, the prescribing rheumatologist chose to appeal an initial rejection of the b/tsDMARD prescription, which led to the third-party payer ultimately approving the prescription; these occurences were recorded in each case, though such patients were not considered as being approved for purposes of our analysis since additional/significant administrative effort was required to obtain their initial b/tsDMARD prescription.
There were 150 RA patients in our practice who had a PrismRA test performed in 2021- 2022. 65 patients (43%) had a PrismRA test result indicating a high or very high likelihood of inadequate response to TNFi therapy. Of these, 41 patients were subsequently prescribed a non-TNFi b/tsDMARD by their rheumatologist, which defined the target population for analysis as shown in Figure 1. Case characteristics of these 41 patients are summarized in Table 1. Patients were predominantly female (73%) and Caucasian (95%). Mean age at the time of PrismRA-guided non-TNFi b/tsDMARD prescription was 60 ± 13 years.
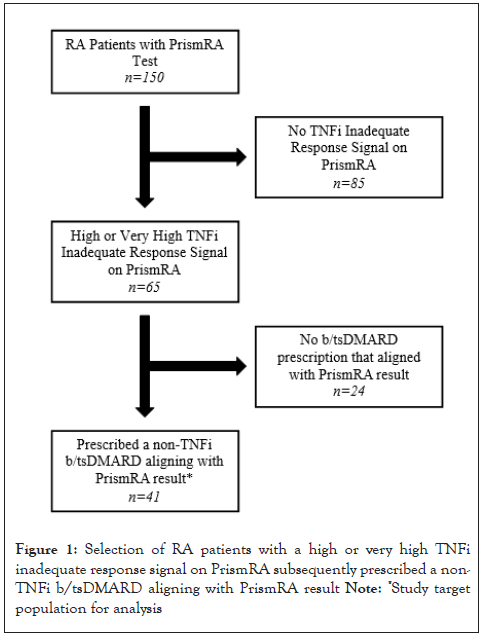
Figure 1: Selection of RA patients with a high or very high TNFi
inadequate response signal on PrismRA subsequently prescribed a non-TNFi b/tsDMARD aligning with PrismRA result
Note: *Study target
population for analysis
| Total cases (n=41) | |
|---|---|
| Age at time of non-TNFi b/tsDMARD prescription that aligned with PrismRA, mean ± SD (years) |
60 ± 13 |
| Sex, n (%) | |
| Female | 30 (73%) |
| Male | 11 (27%) |
| Race/Ethnicity, n (%) | |
| Caucasian | 39 (95%) |
| African American | 2 (5%) |
| PrismRA inadequate response signal | |
| High | 32 (78%) |
| Very high | 9 (22%) |
Table 1: Case characteristics of RA patients prescribed a non-TNFi b/tsDMARD that aligned with PrismRA test results
Outcomes of payer approval/rejection determinations are described in Table 2, partitioned by payer and TNFi exposure status; additional details of patients’ third-party payer status are provided in Table 3. 28 patients were commercially insured or had a health plan provided by the Veteran’s Administration (VA). Of these, 18 patients (64%) had their non-TNFi b/tsDMARD prescription approved by their third-party payer without requiring appeal or peer-to-peer discussion. Among the 10 commercially insured/ VA patients who had their non-TNFi b/tsDMARD prescription rejected by their third-party payer, 4 patients had the initial rejection overturned on appeal when we shared the patients’ PrismRA result with their third-party payer. 13 patients had an Original Medicare or Medicare Advantage Plan. Of these, 11 patients (85%) had their non-TNFi b/tsDMARD prescription approved and were able to start treatment.
| Payer | Approved | Denied |
|---|---|---|
| All payers | 29 (71%) | 12 (29%)* |
| TNFi naïve N=21 |
16 (76%) | 5 (24%) |
| TNFi exposed N=20 |
13 (65%) | 7 (35%)* |
| Commercial/VA | 18 (64%) | 10 (36%)* |
| TNFi naïve N=11 |
8 (73%) | 3 (27%) |
| TNFi exposed N=17 |
10 (59%) | 7 (41%)* |
| Medicare | 11 (85%) | 2 (15%) |
| TNFi naïve N=10 |
8 (80%) | 2 (20%) |
| TNFi exposed N=3 |
3 (100%) | 0 |
Note: * 4 of these patients had their initial non-TNFi b/tsDMARD prescription rejection overturned on appeal
Table 2: RA patients prescribed a non-TNFi b/tsDMARD that aligned with PrismRA test results by third-party payer and TNFi exposure status
| Payer | Patients | Approved |
|---|---|---|
| Commercial/VA | 28 | 18 (64%) |
| Anthem blue cross blue shield | 15 | 9 (60%) |
| Cigna | 3 | 1 (33%) |
| Veteran’s administration | 3 | 2 (66%) |
| United healthcare | 3 | 3 (100%) |
| Humana | 2 | 1 (50%) |
| Aetna | 2 | 2 (100%) |
| Medicare | 13 | 11 (85%) |
| Original medicare with medicare plan supplement | 10 | 10 (100%) |
| Medicare advantage | 3 | 1 (33%) |
Table 3: Details of third-party payer status for RA patients prescribed a non-TNFi b/tsDMARD that aligned with PrismRA test results
Altogether, 29 of 41 patients (71%) in our practice who had a PrismRA test result with a high or very high likelihood of inadequate response to TNFi therapy and were subsequently prescribed a non-TNFi b/tsDMARD had the selected treatment (or in-class alternative) approved by the third- party payer without need of appeal or peer-to-peer discussion.
Within the group of 28 patients with commercial insurance or a VA health plan, 11 patients were naïve to all TNFi medications. 8 of these patients (73%) had their non-TNFi b/tsDMARD approved by their third-party payer without need of appeal or peer-to-peer discussion. Of the 17 patients previously exposed to TNFi therapy prior to their PrismRA result, 10 patients (59%) had their non- TNFi b/tsDMARD approved by their third-party payer without need of appeal or peer-to-peer discussion.
Within the group of 13 Original Medicare/Medicare Advantage patients, 10 patients were naïve to all TNFi medications. 8 of these patients (80%) had their non-TNFi b/tsDMARD approved and were able to start treatment. Of 3 patients previously exposed to TNFi therapy, all 3 patients (100%) had their non-TNFi b/ tsDMARD approved by their third-party payer without need of appeal or peer-to-peer discussion.
Altogether, 16 of 21 patients (76%) in our practice who were naïve to TNFi therapy, had a PrismRA test result indicating a high or very high likelihood of inadequate response to TNFi therapy, and were subsequently prescribed a non-TNFi b/tsDMARD, also had their prescriber’s intended non-TNFi b/tsDMARD therapy (or an in-class alternative) approved by their third-party payer without need of appeal or peer-to-peer discussion. Meanwhile, 13 of 20 patients (65%) in our practice who had been previously exposed to TNFi therapy, had a PrismRA test result indicating a high or very high likelihood of inadequate response to TNFi therapy and were subsequently prescribed a non-TNFi b/tsDMARD, also had their prescriber’s intended non-TNFi b/tsDMARD therapy (or in- class alternative) approved by their third-party payer without need of appeal or peer-to-peer discussion.
Until recently, personalized medicine utilizing patient-specific factors including genomic data in order to match individual patients to best therapies had been sorely lacking in the management of rheumatoid arthritis. This was in spite of the growing incorporation of personalized medicine within other healthcare fields, particularly oncology, in which oncolytic regimens have been tailored to specific genetic mutations underlying an individual patient’s cancer [17-19]. More recently, in the field of psychiatry, genetic testing and biomarkers have been deployed to predict clinical response or adverse effects to specific anti- depressant agents [20-22]. The PrismRA test represents a new and exciting extension of personalized medicine into the management of RA by predicting inadequate response to TNFi therapy in RA patients. As such, PrismRA equips rheumatologists with a clinically validated biomarker test for bypassing TNFi therapy as a first-line b/tsDMARD in certain RA patients failing csDMARD therapy, thereby facilitating more rapid and effective disease control for such patients when they can be matched to a non-TNFi b/tsDMARD.
The clinical benefits of personalized medicine tools including PrismRA can only be fully realized, however, if ST protocols partner with such advancements to enable patients to access most effective therapy. Currently, ST protocols are often perceived by rheumatologists to restrict access to non-TNFi agents as first-line b/tsDMARDs. Contrary to this perception, our study revealed that the majority (71%) of RA patients in our practice who had a PrismRA result indicating high or very high likelihood of inadequate response to TNFi therapy were not prevented from having a non-TNFi b/tsDMARD immediately approved by their third party payer without need of appeal or peer-to-peer discussion.
While the intent of this retrospective study was not to document the approval/rejection patterns of individual payers but rather to document the experience of an individual, real-world rheumatology practice amongst a broad payer mix, it is nonetheless true that specific ST protocols are unique to each health plan. Consequently, approval of non-TNFi agents as first-line b/tDMARDs could vary significantly between third-party payers. To help investigate this, we divided our patient cohort by payer status according to whether the patient was commercially insured or had a health plan through the VA, and those patients with an Original Medicare or Medicare Advantage plan. This revealed that a still significant majority of commercially insured/VA patients (64%) had a non-TNFi agent immediately approved following their PrismRA result. Patients with an Original Medicare or Medicare Advantage plan had an even higher success rate (85%) of approval of a non-TNFi agent following their PrismRA result.
Given that Medicare makes prescription coverage determinations based upon medical necessity that can still leave patients with unaffordable copays, our analysis ensured that patients with an Original Medicare or Medicare Advantage plan had to receive/start their prescribed non-TNFi b/tsDMARD in order to be considered approved for purpose of our analysis.
Of course, a patient’s previous use of b/tsDMARD therapies may influence their payer’s approval/rejection decision of a non-TNFi b/tsDMARD according to the health plan’s specific ST protocol. To address this potential confounding variable, we analyzed our patient cohort according to whether patients were TNFi-naïve or TNFi-exposed prior to their PrismRA test result. This showed that a significant majority of both TNFi-naïve patients (76%) and TNFiexposed patients (65%) had their prescriber’s intended non-TNFi b/tsDMARD therapy (or in-class alternative) approved by their third-party payer without need of appeal or peer-to-peer discussion, with no significant difference between these groups.
Limitations of this study include that this was an analysis of a single, private rheumatology practice located in the midwest/southeast portion of the U.S., thus potentially reducing its generalizability. Nevertheless, our cohort included a diverse set of health plans and third-party payer groups including payers with national scope such as the VA and Medicare, thereby increasing the applicability of our findings to rheumatology practices in different regions of the country. Of note, we combined patients with commercial insurance and patients with a VA health plan in our analysis due to the fact that VA health plans function similarly to commercial payers in regard to their ST protocols and formulary preferences. Other limitations include a relatively small number of patients, including those who were naïve to TNFi therapy and therefore most susceptible to health plan ST policy restrictions; as well as a relatively homogenous ethnic/racial makeup of our patient cohort. Despite these limitations, our cohort stemming from 150 RA patients with a PrismRA test, including 65 patients with a high or very high PrismRA result, represents the largest real-world population to-date to undergo this type of analysis, detailing the prescribing and approval outcomes of bypassing TNFi therapy as a first- line b/tsDMARD using a personalized medicine approach.
While ST protocols presented less of an obstacle to implementing PrismRA results in our patients than we had hypothesized, recent and ongoing legislative action by federal and state government is further reducing opposition to personalized medicine posed by ST policies within rheumatology and other healthcare fields [23]. At present, 35 states have enacted laws limiting ST restrictions on medications deemed clinically-indicated by a prescribing healthcare provider [24]. In Kentucky where our practice is located, a state bill became effective on January 1st, 2023, which mandates that a patient’s insurer, health plan or pharmacy benefit manager grant exceptions to ST protocols under a variety of circumstances, including when a preferred formulary medication is “expected to be ineffective” [25]. This category of exception relates directly to the intended purpose of PrismRA for predicting inadqueate response to TNFi therapy in RA patients. Of note, the patients included in our analysis had a PrismRA performed and subsequent b/ tsDMARD prescribed prior to January 1st, 2023, and thus were not impacted by the recent Kentucky ST legislation. In future work, we intend to perform a similar analysis of RA patients in our practice whose PrismRA results and subsequent b/tsDMARD prescriptions occur after this recent Kentucky legislation became effective in order to identify remaining barriers to personalized medicine that are negatively impacting our patients.
With the commercial availability of PrismRA, rheumatologists have been equipped with a personalized, clinically validated biomarker test to identify which RA patients within their practice might benefit from first-line use of a non-TNFi b/tsDMARD after failing csDMARD therapy. Prior to this analysis, third-party payers’ ST protocols were a perceived obstacle for bypassing TNFi medications as first-line b/tsDMARD therapy in RA patients who had a PrismRA result showing high likelihood of inadequate response to TNFi therapy. This study of a real-world, community rheumatology practice showed that a significant majority of RA patients (71%) with a PrismRA result indicating high likelihood of inadequate response to TNFi therapy had their prescriber’s intended non-TNFi b/tsDMARD therapy (or in-class alternative) approved by their third-party payer without need of appeal or peer-to-peer discussion. The rate of non- TNFi b/tsDMARD approval by payers did not significantly differ between patients who were TNFi-naïve or TNFi-exposed prior to their PrismRA result. Altogether, our study results suggest that even in the current landscape of widespread ST adoption by health plans and thirdparty payers, rheumatologists are not impeded from implementing PrismRA results in a significant majority of their RA patients who are predicted to not respond to TNFi therapy.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Boone J, Crump G, Walters R (2023) Confronting Step Therapy in Rheumatoid Arthritis: A Rheumatology Practice's Experience Using PrismRA to Bypass Ineffective TNF Inhibitor Therapies. Rheumatology (Sunnyvale). 13: 363
Received: 27-Jul-2023, Manuscript No. RCR-23-25835; Editor assigned: 31-Jul-2023, Pre QC No. RCR-23-25835 (PQ); Reviewed: 16-Aug-2023, QC No. RCR-23-25835; Revised: 23-Aug-2023, Manuscript No. RCR-23-25835 (R); Published: 30-Aug-2023 , DOI: 10.35841/2161-1149.23.13.363
Copyright: © 2023 Boone J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.