
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Short Communication - (2023)Volume 13, Issue 3
The ubiquitous contamination of food products with Alternaria Toxins (ATs) and Ergot Alkaloids (EAs) often goes unnoticed due to the lack of clear assessments of their human toxicity. To address this issue, a fast and Liquid Chromatography-Mass Spectrometry (LC-MS) friendly method was developed for the accurate and robust analysis of a total of 37 mycotoxins, including ATs and EAs, in various food commodities. This analytical workflow was established through a thorough examination of different types of LC bonding chemistries, sample extraction conditions, and calibration methodologies to ensure accuracy and precision in the measurement.
Mycotoxins; Alternaria toxins; Ergot alkaloids; Aspergillus
Mycotoxins are secondary metabolites produced by several fungal species of the genus Aspergillus (aflatoxins and ochratoxins), Claviceps (Ergot Alkaloids (EAs)), Fusarium (beauvericin, fumonisins, trichothecenes, and zearalenone), Penicillium (citrinin, patulin), and Alternaria (alternariol, tentoxin, and tenuazonic acid), among others. While ATs and EAs are not currently regulated in the United States, the European Union has established the regulated levels for EAs and is in the process of discussing the maximum permitted levels for ATs [1-3]. EAs contamination is common in cereal crops and wild grasses [4], whereas ATs contamination is prevalent in fruits, vegetables, and cereals [5]. Their co-occurrence in grain-based foods highlights the need for developing an analytical method for the simultaneous analysis of ATs and EAs in such food and feed matrices. However, due to their distinct chemical properties, developing a comprehensive method for simultaneous analysis of multi-class mycotoxins, including emerging ATs and EAs, has been a significant challenge. For liquid chromatography, specific analytical methods using high pH conditions are usually required for the analysis of ATs and EAs to either gain acceptable peak shapes or enable the chromatographic separation of epimers [6-9]. These practices, however, have hindered the simultaneous analysis of these two groups of compounds with other major regulated mycotoxins.
Among more than 70 identified ATs, the five most important ones are altenuene, alternariol, alternariol monomethylether, tentoxin, and tenuazonic acid [10]. Of more than 40 known EAs, the European Union lists six EAs, including ergocornine, ergocristine, ergocryptine, ergometrine, ergosine, and ergotamine, along with their isomeric-inine epimers, as regulated EAs. The guidance is to quantify these 12 EAs separately and sum them up to a total EAs level [11]. Therefore, it is necessary to chromatographically resolve all 12 epimeric EAs for unambiguous and accurate quantification. This study aimed to develop a simplified and unique method for analyzing ATs and EAs together with other major regulated mycotoxins. The method’s suitability was evaluated in terms of linearity, specificity, accuracy, precision, and adaptability.
The chromatographic performance was evaluated on Biphenyl, FluoroPhenyl, and C18 stationary phases bonded to superficially porous particles. It was observed that the baseline separation of six EAs and their epimers could only be accomplished on the Biphenyl column under regular acidic conditions.
Similar to other types of multi-compound analysis in food by LCMS/ MS, accurate quantification of multi-mycotoxins in various food commodities requires mitigating the matrix effect. The most direct approach to correct matrix effects is to use stable isotopes as internal standards for quantitative standard calibration. This approach, however, may not be applicable to most multi-mycotoxin analyses due to the lack of isotopicallylabeled compounds for certain analytes. Therefore, the matrixmatched external standard calibration was implemented in this study to equally compensate for the matrix effect of calibration standards and sample solutions for accurate quantification.
Four different food commodities (baby wheat cereal, peanut, tomato puree, and blended flour) were chosen for method validation to demonstrate the applicability of this analytical method across a wide range of food types. Sample extraction was performed using a formic acid-acidified 80:20 Acetonitrile:Water solution followed by extract dry-down and reconstitution in a 50:50 Water:Methanol solution for analysis on a Biphenyl LC column. Chromatographic analysis was performed under LC-MS friendly conditions with a short 11-minute cycle time. The chromatographic and mass spectrometry (Waters Xevo TQ-S) conditions are provided in Table 1, and the resulting chromatogram is shown in Figure 1. Method accuracy and precision were evaluated with samples fortified at 5, 50, and 200 μg/kg. Accurate quantification was achieved using matrixmatched calibration standards at the range of 0.4 to 400 μg/kg. The recoveries of all mycotoxins (except citrinin) in fortified samples were from 70% to 120%, and the relative standard deviation was less than 20% (Table 2). More detailed sample preparation procedures and results can be found in the study of Liang, et al. [12].
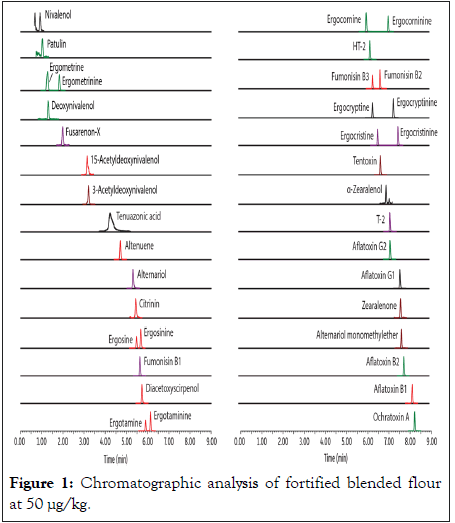
Figure 1: Chromatographic analysis of fortified blended flour at 50 μg/kg.
| Chromatographic and mass spectrometry conditions | ||
|---|---|---|
| Analytical column | Raptor Biphenyl 2.7 µm, 100 mm x 2.1 mm | |
| Guard column | Raptor Biphenyl EXP guard colum cartridge 2.7 um, 5 mm x 2.1 mm | |
| Mobile phase A | 0.05% formic acid in water | |
| Mobile phase B | 0.05% formic acid in methanol | |
| Gradient | Time (min) | %B |
| 0 | 25 | |
| 5 | 50 | |
| 9 | 100 | |
| 9.01 | 25 | |
| 11 | 25 | |
| Flow rate | 0.4 mL/min | |
| Injection volume | 5 µL | |
| Column temperature | 60°C | |
| Capillary voltage | 0.7 kV | |
| Gas flow | 1000 (L/Hr) Disolvation; 150 (L/Hr) Cone; 7.0 (bar) Nebuliser | |
| Desolvation temperature | 500°C | |
Table 1: The chromatographic and mass spectrometry conditions.
| Average Recovery (RSD, %) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baby wheat cereal | Peanut | Tomato puree | Blended flour | |||||||||
| Concentration, µg/kg | 5 | 50 | 200 | 5 | 50 | 200 | 5 | 50 | 200 | 5 | 50 | 200 |
| Aflatoxin B1 | 105 (4.8) | 100 (3.0) | 79.8 (2.6) | 98.2 (6.4) | 97.0 (5.2) | 89.0 (5.7) | 92.7 (3.8) | 97.6 (5.2) | 103 (3.0) | 101 (2.8) | 95.5 (1.3) | 89.0 (1.5) |
| Aflatoxin B2 | 110 (1.4) | 109 (2.8) | 106 (2.3) | 102 (5.8) | 99.3 (4.7) | 91.3 (2.9) | 91.7 (4.2) | 93.3 (0.9) | 94.7 (0.4) | 100 (2.3) | 101 (0.9) | 88.7 (1.3) |
| Aflatoxin G1 | 105 (6.1) | 107 (1.7) | 102 (2.1) | 98.2 (4.2) | 97.3 (3.2) | 91.2 (4.1) | 91.3 (1.9) | 92.2 (3.6) | 93.3 (2.5) | 99.3 (1.7) | 100 (1.6) | 93.6 (2.2) |
| Aflatoxin G2 | 108 (3.0) | 109 (1.3) | 104 (2.2) | 104 (5.3) | 102 (3.8) | 93.5 (1.9) | 86.8 (8.3) | 96.4 (2.5) | 98.5 (2.5) | 98.7 (3.1) | 102 (2.6) | 94.5 (2.0) |
| Ochratoxin A | 109 (1.8) | 108 (2.1) | 94.5 (1.5) | 102 (1.9) | 101 (1.1) | 97.7 (0.9) | 90.9 (3.5) | 93.8 (3.3) | 101 (5.9) | 98.1 (1.6) | 98.2 (1.3) | 82.8 (1.7) |
| 3- + 15-Acetyldeoxynivalenol | 104 (6.3) | 108 (1.8) | 104 (3.3) | 101 (6.5) | 95.9 (5.8) | 91.0 (4.4) | 91.9 (4.3) | 98.1 (2.7) | 95.0 (1.8) | 98.4 (5.2) | 101 (2.9) | 100 (0.9) |
| Deoxynivalenol | 112 (4.0) | 102 (2.6) | 95.7 (1.3) | 98.1 (3.5) | 93.7 (4.8) | 88.2 (3.4) | - | 90.3 (6.4) | 94.5 (2.6) | 102 (3.5) | 97.5 (2.6) | 96.9 (0.8) |
| Diacetoxyscirpenol | 105 (4.0) | 107 (1.5) | 103 (1.2) | 93.2 (4.3) | 95.4 (3.9) | 93.8 (5.0) | 90.9 (3.8) | 94.5 (4.7) | 94.0 (1.9) | 98.1 (6.3) | 101 (3.1) | 98.7 (1.8) |
| Fumonisin B1 | 94.3 (4.6) | 94.0 (2.8) | 92.3 (2.6) | 87.2 (3.1) | 88.2 (4.5) | 87.8 (6.6) | 91.8 (3.6) | 91.5 (1.9) | 91.9 (0.7) | 100 (3.2) | 99.6 (1.7) | 96.1 (1.2) |
| Fumonisin B2 | 93.3 (4.1) | 95.1 (4.8) | 90.3 (2.9) | 95.4 (4.7) | 92.5 (2.3) | 88.8 (3.9) | 89.9 (4.1) | 92.9 (2.3) | 92.4 (0.8) | 104 (2.7) | 99.6 (1.4) | 94.4 (1.6) |
| Fumonisin B3 | 91.8 (4.9) | 94.6 (4.9) | 91.6 (3.1) | 90.6 (2.7) | 90.1 (3.8) | 87.7 (4.7) | 91.1 (3.6) | 93.1 (1.8) | 91.9 (0.9) | 104 (2.2) | 99.9 (1.4) | 95.9 (1.2) |
| Fusarenon-X | 99.0 (3.9) | 100 (2.9) | 103 (2.8) | 86.9 (7.0) | 90.3 (11.0) | 88.3 (10.1) | - | 92.0 (6.8) | 94.3 (1.9) | 101 (3.8) | 100 (3.7) | 98.3 (1.6) |
| HT-2 | 110 (2.4) | 111 (1.4) | 108 (1.1) | 100 (2.7) | 100 (2.0) | 94.3 (3.0) | 96.8 (3.1) | 96.1 (2.1) | 99.0 (1.4) | 101 (1.6) | 103 (2.2) | 98.3 (1.3) |
| Nivalenol | - | - | - | - | 98.3 (6.2) | 89.0 (3.6) | - | 92.5 (4.5) | 93.7 (5.0) | - | 95.5 (4.7) | 92.9 (2.3) |
| T-2 | 111 (2.1) | 110 (1.8) | 108 (2.8) | 99.1 (2.7) | 101 (1.7) | 95.9 (2.1) | 92.0 (6.3) | 94.7 (1.3) | 98.6 (1.5) | 102 (1.3) | 103 (1.3) | 96.9 (1.3) |
| α-Zearalenol | 100 (4.9) | 102 (5.2) | 90.1 (5.8) | 89.2 (8.1) | 93.6 (5.5) | 94.7 (3.4) | 97.7 (3.2) | 88.9 (4.2) | 90.0 (3.4) | 96.9 (3.7) | 99.0 (3.6) | 95.0 (3.3) |
| Zearalenone | 110 (6.7) | 110 (3.0) | 105 (3.7) | 98.3 (7.3) | 97.4 (2.8) | 91.3 (1.5) | 95.0 (4.5) | 93.6 (2.2) | 95.7 (2.0) | 101 (3.8) | 102 (2.1) | 92.3 (1.4) |
| Citrinin | 26.1 (9.2) | 26.6 (3.1) | 30.1 (3.8) | 24.1 (8.7) | 25.1 (1.9) | 25.8 (3.5) | 71.9 (4.7) | 76.4 (1.6) | 77.1 (1.7) | 32.3 (3.5) | 32.2 (6.3) | 35.8 (4.5) |
| Patulin | 106 (4.6) | 95.6 (5.6) | 89.2 (5.1) | 88.8 (12.0) | 83.6 (9.0) | 86.0 (7.2) | - | 98.9 (3.6) | 103 (4.5) | 93.6 (4.4) | 86.1 (3.1) | 92.2 (2.9) |
| Alternariol | 108 (4.1) | 108 (1.6) | 104 (1.0) | 94.2 (3.4) | 95.4 (2.4) | 96.2 (2.7) | 89.3 (4.6) | 91.8 (2.5) | 91.4 (1.3) | 98.4 (2.3) | 101 (2.5) | 96.3 (3.2) |
| Alternariol monomethylether | 108 (4.1) | 109 (2.2) | 99.3 (2.7) | 93.5 (3.3) | 93.5 (3.7) | 89.8 (2.4) | 91.3 (6.6) | 88.7 (5.1) | 93.9 (3.9) | 104 (2.9) | 101 (1.7) | 93.7 (1.9) |
| Altenuene | 110 (2.1) | 109 (2.1) | 105 (2.1) | 99.6 (2.0) | 99.5 (1.2) | 95.4 (1.2) | 98.4 (3.4) | 92.4 (2.1) | 92.8 (1.8) | 101 (2.9) | 101 (3.1) | 98.2 (0.5) |
| Tentoxin | 111 (3.6) | 109 (2.5) | 103 (1.4) | 104 (2.9) | 101 (1.1) | 95.3 (1.4) | 92.5 (6.2) | 94.2 (2.2) | 95.8 (1.4) | 104 (4.2) | 105 (2.1) | 98.2 (1.9) |
| Tenuazonic acid | - | 85.8 (1.7) | 87.4 (6.3) | 92.5 (4.7) | 91.0 (2.1) | 88.5 (2.4) | - | 89.3 (4.1) | 88.5 (2.0) | - | 92.5 (8.8) | 90.0 (9.5) |
| Ergocornine | 109 (1.5) | 109 (1.4) | 102 (1.3) | 93.8 (3.5) | 93.2 (4.4) | 91.2 (3.3) | 91.5 (3.0) | 93.1 (1.9) | 92.9 (0.6) | 102 (2.5) | 101 (1.9) | 97.6 (1.7) |
| Ergocorninine | 109 (3.0) | 109 (2.0) | 101 (1.9) | 105 (3.0) | 104 (2.4) | 99.5 (3.1) | 89.9 (3.8) | 92.3 (2.2) | 92.5 (3.1) | 101 (2.5) | 102 (2.6) | 95.7 (2.4) |
| Ergocristine | 108 (3.1) | 108 (2.9) | 101 (4.4) | 92.1 (3.8) | 91.7 (5.1) | 92.0 (2.2) | 91.3 (2.9) | 94.2 (2.0) | 94.3 (0.8) | 101 (1.7) | 99.8 (2.0) | 96.7 (1.8) |
| Ergocristinine | 106 (3.5) | 105 (1.4) | 101 (0.8) | 102 (4.8) | 104 (4.3) | 102 (4.6) | 91.6 (5.9) | 94.4 (1.8) | 95.6 (2.7) | 102 (2.9) | 102 (3.0) | 99.3 (4.5) |
| Ergocryptine | 107 (2.0) | 109 (1.9) | 104 (3.4) | 95.0 (3.0) | 94.7 (4.1) | 92.1 (1.7) | 90.1 (3.0) | 93.5 (2.2) | 93.2 (0.7) | 99.5 (2.7) | 99.9 (1.2) | 97.4 (1.4) |
| Ergocryptinine | 106 (1.7) | 108 (2.0) | 101 (1.1) | 103 (5.3) | 105 (4.0) | 101 (4.2) | 91.1 (4.3) | 95.1 (1.5) | 98.1 (1.6) | 101 (2.0) | 101 (1.8) | 95.4 (1.9) |
| Ergometrine | 92.8 (7.3) | 90.0 (4.2) | 88.3 (3.6) | 101 (2.3) | 96.2 (2.6) | 86.7 (1.9) | 90.7 (3.6) | 88.9 (6.1) | 87.6 (3.5) | 101 (1.8) | 99.7 (3.2) | 95.3 (1.3) |
| Ergometrinine | 101 (4.2) | 99.1 (1.9) | 94.3 (0.7) | 93.2 (4.3) | 95.5 (1.7) | 89.1 (2.2) | 90.6 (3.9) | 90.1 (4.4) | 89.7 (1.9) | 100 (3.5) | 98.5 (1.9) | 91.1 (1.9) |
| Ergosine | 108 (2.6) | 106 (5.6) | 101 (3.2) | 90.8 (2.0) | 91.8 (2.2) | 89.2 (2.6) | 91.7 (2.2) | 90.4 (3.1) | 90.3 (1.5) | 99.9 (2.7) | 99.1 (3.0) | 98.2 (1.1) |
| Ergosinine | 111 (1.8) | 109 (0.9) | 103 (1.1) | 100 (1.1) | 102 (2.0) | 97.7 (2.2) | 92.7 (1.4) | 93.6 (2.5) | 93.8 (0.9) | 99.2 (2.8) | 98.4 (2.8) | 97.5 (1.0) |
| Ergotamine | 109 (1.9) | 108 (1.7) | 102 (2.8) | 91.0 (2.8) | 92.6 (2.8) | 89.8 (3.6) | 91.1 (2.2) | 90.6 (3.7) | 90.7 (1.3) | 101 (2.9) | 100 (3.1) | 96.4 (2.2) |
| Ergotaminine | 109 (1.0) | 109 (0.7) | 101 (0.6) | 98.2 (2.0) | 101 (1.5) | 96.6 (1.3) | 93.6 (3.5) | 94.7 (1.7) | 94.5 (0.6) | 101 (2.3) | 99.7 (1.3) | 97.1 (1.5) |
Table 2: Accuracy and precision analysis of fortified food samples.
This study established a workflow for the simultaneous determination of Alternaria toxins, ergot alkaloid epimers, and other major mycotoxins produced by the fungal genus of Aspergillus, Fusarium, and Penicillium. This approach can be applied to quantify multiple mycotoxins in a wide range of food commodities. A matrix-matched calibration was implemented to compensate for inherent matrix effects. The majority of analytes could be measured at a concentration as low as 0.4 μg/kg, which was satisfactory to meet the regulatory requirements.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Liang SH (2023) Comprehensive Mycotoxin Analysis: Adding Alternaria Toxins and Ergot Alkaloids to Multi-Mycotoxin Analysis in Various Food Commodities. J Clin Toxicol. 13:533.
Received: 26-May-2023, Manuscript No. JCT-23-24410; Editor assigned: 29-May-2023, Pre QC No. JCT-23-24410 (PQ); Reviewed: 12-Jun-2023, QC No. JCT-23-24410; Revised: 19-Jun-2023, Manuscript No. JCT-23-24410 (R); Published: 26-Jun-2023 , DOI: 10.35248/2161-0495.23.13.533
Copyright: © 2023 Liang SH. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.