Research - (2023)Volume 7, Issue 1
Background: Heart failure has been a rising concern in Tanzania. New drugs have been introduced, including the group of drugs called Angiotensin Receptor Neprilysin Inhibitors (ARNI), but due to their high cost, Angiotensin-Converting Enzyme Inhibitors (ACEIs) and Angiotensin Receptor Blockers (ARBs) have been mostly used in Tanzania. However, according to our knowledge, the efficacy comparison of the two groups is yet to be studied in Tanzania. The aim of this study was to compare the efficacy of ACEIs and ARBs among patients with heart failure.
Methodology: This was a hospital-based prospective cohort study done at Jakaya Kikwete Cardiac Institution (JKCI), Tanzania, from June to December 2020. Consecutive enrollment was done until fulfilling the inclusion criteria. Clinical details were measured at baseline. We assessed the relationship between ARBs and ACEI users with N-terminal pro-Brain Natriuretic Peptide (NT-proBNP) levels at admission and at 1-month follow-up using a chi-square test. A Kaplan-Meier curve was used to estimate the survival time of the two groups.
Results: 155 HF patients were enrolled, with a mean age of 48 years, whereby 52.3% were male, and their mean Left Ventricular Ejection Fraction (LVEF) was 52 (33.5%) heart failure patients were on ACEIs, 57 (36.8%) on ARBs, and 46 (29.7%) were neither using ACEIs nor ARBs. At least half of the patients did not receive a Guideline Directed Medical Therapy (GDMT), with only 82 (52.9%) receiving a GDMT. A drop in NT-proBNP levels was observed during admission and at 1 month follow-up on both groups, from 6389.2 pg/ml to 4000.1 pg/ml for ARB users and 5877.7 pg/ml to 1328.2 pg/ml for the ACEIs users. There was no statistical difference between the two groups when estimated by the Kaplan-Meier curve, though, more deaths were observed in those who were neither on ACEIs nor ARBs, with a calculated P value of 0.01.
Conclusion: This study demonstrates that ACEIs have more efficacy and overall better clinical outcome than ARBs, but this should be taken under the patient-based case, considering the side effects of ACEIs and patients’ adherence.
Angiotensin converting enzyme inhibitors; Angiotensin receptor blockers; Guideline direct medical therapy; N-terminal pro-brain natriuretic peptide
Heart failure (HF) is a global public health problem, affecting approximately 26 million people worldwide [1]. The increase of none communicable diseases e.g. Hypertension (HTN), Diabetes Mellitus (DM), dyslipidemia and life style changes in Low- and Middle-Income Countries (LMICs) has led to an increment of heart failure cases, as documented in a meta-analysis done in Africa [2]. Natriuretic peptides have been used as diagnostic and prognostic biomarkers for heart failure [3]. Due to the burden of heart failure, every year different heart failure societies sit and discuss new regimens to improve the quality of life and heart remodeling. New drugs have been introduced including the group of drugs called Angiotensin receptor Neprilysin Inhibitor (ARNI); Sacubril-Valsartan as published in the PARADGM TRIAL comparing it with Angiotensin Converting Eenzyme Inhibitors (ACEIs). These drugs have shown more effectiveness in HF with reduced ejection fraction compared to ACEIs [4]. Long before this drug was introduced, already HF was responsible for more death in LMICs compared to western countries, mostly due to failure in being prescribed Guideline Directed Medical Therapy (GDMT) [5]. In Tanzania, HF has been more prevalent not only on middle class population but also in low class population, whereby not only they cannot afford drugs like in Angiotensin Receptor Neprilysin Inhibitor (ARNI) group, but also availability of it, especially in remote areas is difficult [6]. Drugs in group of Angiotensin Receptor Blockers (ARBs), have been widely used to these patients than ACEIs, mostly due to adherence, mostly taken as a once daily dose, compared to ACEIs that are mostly taken twice or thrice a day, also considering the side effects of ACEIs, that is mostly cough. A meta-analysis study has shown that ACEIs have been more effective compared to ARBs [7], bringing the question, could more patients with HF in Tanzania benefit from the use of ACEIs rather than ARBs, should our practice change? However, no studies have compared the efficacy of the two groups by assessing them with the levels of natriuretic peptides. In response to this problem, we therefore used the natriuretic peptide plasma NT pro-BNP and assessed it with the two groups during admission and at 1-month follow-up, and estimated the survival rate between the two groups within the 6-month study period in a Tanzanian population.
Objectives
This study aimed to compare the efficacy of ACEIs and ARBs in patients with heart failure and specifically to associate between plasma NT pro-BNP levels with ARB and ACEIs, and to determine the clinical progression between the two groups. The flow chart of patients enrolment is shown in Figure 1.
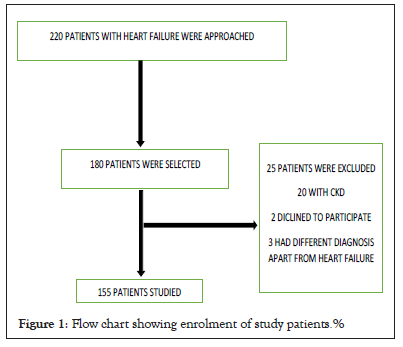
Figure 1: Flow chart showing enrolment of study patients.%
Study Design
This was a hospital-based prospective cohort study.
Study Settings
The study was done at Jakaya Kikwete Cardiac Institution (JKCI), Ilala district, Dar-es Salaam, Tanzania. JKCI is one of a national tertiary level referral hospital basing mainly on cardiac diseases, both medical and surgical. It has a capacity of 103 beds serving about 800 patients per week. It receives patients from the 5 municipalities in Dar es Salaam (Ilala, Kinondoni, Kigamboni, Ubungo and Temeke) but also receives referral cases from the other 26 regions of the country. It also serves as a teaching hospital for Muhimbili University of Health and Allied Sciences (MUHAS). The study was conducted for a period of six months (June 2020-December 2020.) The investigator on a daily basis visited the cardiac wards to look for any patient with a diagnosis of HF as per attending physicians’ diagnosis and confirmed the diagnosis using the Framingham criteria. Consecutively patients with HF diagnosis were then recruited in the study. One research assistant was engaged to help with data collection to recruit all patients with HF. Patients were given an appointment date to visit the clinic after 1 month from their discharge date. During the visit, a detailed history of any cardiovascular events or change in medication was taken, and a thorough physical examination was done to determine the patient’s clinical status and a follow up NT-proBNP was taken. To maintain contact, patients were called through their mobile phone during the 1-month period post discharge. For patients whose mobile phones were not reached, the investigator determined if the patient was alive or passed away at that time. All alive patients were reminded of their 1-month clinic visit for physical assessment. All information was recorded in the patients’ data collection forms.
Participants
All in-patients who were diagnosed to have HF by the attending physician and admitted, aged 18 years and above, and excluded HF patients with CKD.
Variables
Socio-demographics such as age, gender, level of education, occupation, and residence were noted. The primary outcome was the overall clinical progression at 1-month follow-up and mortality within the 6-month study period. Age, sex and smoking were considered as possible confounders.
Data sources/measurements
After a consecutive sampling, patients were then categorized into two groups, those who were on ARBs and on ACEIs (Figures 2 and 3). Some patients were using neither of the two groups, and were separately grouped. Two different strategies for assessing NT-proBNP were examined: Admission levels and the percent change in NT-proBNP levels from admission to follow up. For all patients, a blood sample was taken within 12 hours of admission and this was considered as admission NT-proBNP. Follow up NT-proBNP was done at 1 month after the baseline test was taken. Other baseline laboratory results like haemoglobin level, creatinine level as well as Blood Urea Nitrogen (BUN) were obtained from patients’ files and recorded in data collection forms. Echocardiogram results were obtained from detailed echocardiogram examination done within the index admission, where the ejection fraction was the main parameter taken though other parameters were also assessed like diastology but was not used in this study.
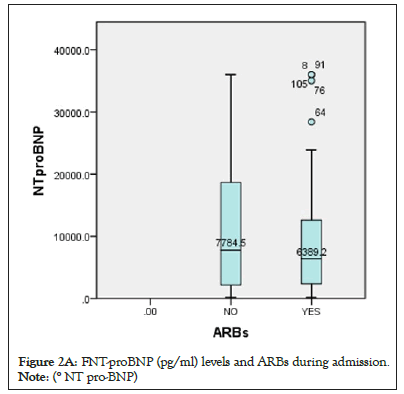
Figure 2a: FNT-proBNP (pg/ml) levels and ARBs during admission.
Note: (° NT pro-BNP)
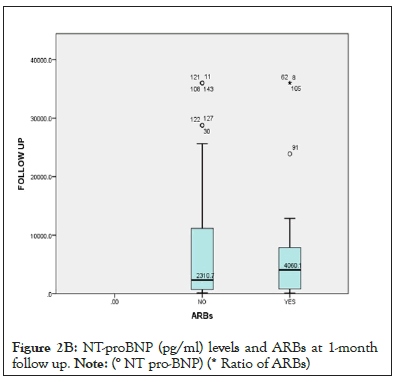
Figure 2b: NT-proBNP (pg/ml) levels and ARBs at 1-month
follow up.
Note: (° NT pro-BNP) (* Ratio of ARBs)
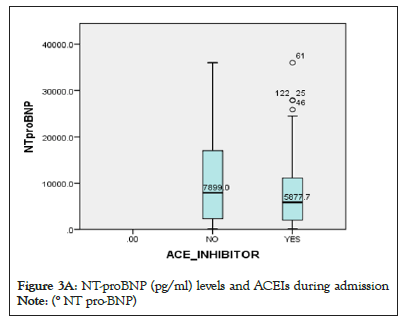
Figure 3a: NT-proBNP (pg/ml) levels and ACEIs during admission.
Note: (° NT pro-BNP)
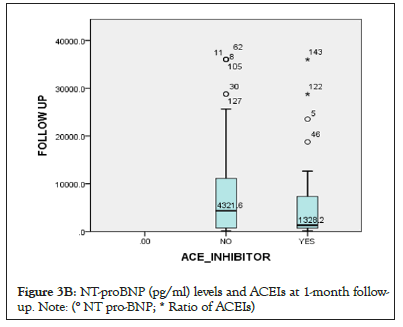
Figure 3b: NT-proBNP (pg/ml) levels and ACEIs at 1-month followup.
Note: (° NT pro-BNP; * Ratio of ACEIs)
Bias: To minimize this, all patient was followed equally, when patients returned to their clinics at 1-month follow-up, all physicians were blinded from the study, where patients were asked whether they used their medications daily. Patients who were from outside the city, were advised to remain in the city for 1 month after being discharged, and for those who didn’t, an arrangement with their general doctor was done, and at 1-month period, we communicated with their doctors for feedback.
Sample size
The sample size was calculated by using the following formula, Nr=Z2p (1-p)/e2 and adjusted for finite population, sample size n, n=nr/(1+(nr-1)/N), hence, the required sample was approximated to 155 patients. The prevalence used from this study was 45%, from the Kelsey study [8] (Table 1).
| Variable | n (%) / mean (SD) |
|---|---|
| Age group (years), n (%) ≤ 25 | 8 (5.2) |
| 26-35 | 40 (25.8) |
| 36-45 | 26 (16.8) |
| 46-60 | 45 (29.0) |
| >60 | 36 (23.2) |
| Mean (SD) age (years) | 48 (16) |
| Males, n (%) | 81 (52.3) |
| Residing in Dar es salaam, n (%) | 108 (69.7) |
| Marital status, n (%) | |
| Single | 30 (19.4) |
| Married | 112 (72.3) |
| Divorced | 9 (5.8) |
| Widowed | 4 (2.6) |
| Education, n (%) | |
| No formal education | 14 (9.0) |
| Primary | 28 (18.1) |
| Secondary | 3 (1.9) |
| High school | 39 (25.2) |
| College | 71 (45.8) |
| Taking alcohol, n (%) | 29 (18.7) |
| Ever smoked cigarettes, n (%) | 19 (10.3) |
| Cardiovascular risk factors, n (%) | |
| Hypertension | 78 (50.3) |
| Diabetes | 31 (20) |
| Rheumatic heart disease | 47 (31.6) |
| Arrhythmias | 23 (15.5) |
| Mean (SD) SBP (mmHg) | 124.8 (20.5) |
| Mean (SD) DBP (mmHg) | 76.1 (13.1) |
| Mean (SD) BMI (kg/m2) | 22.6 (6.3) |
| BMI category, n (%) | |
| Normal | 152 (98.1) |
| Underweight | 23 (14.8) |
| Overweight | 97 (62.6) |
| Obese | 35 (22.6) |
| Mean LVEF (%) | 37.3 (10.7) |
Note: N=Number of patients; SD=Standard Deviation; SBP=Systolic Blood Pressure; DBP=Diastolic Blood Pressure; BMI=Body Mass Index was calculated using height and weight (BMI= weight in kg /height in meters2).
Table 1: Socio-demographic and clinical characteristics of the study patients, N=155.
Statistical methods
Data was entered on IBM SPSS version 26. Descriptive statistics were analysed using frequency for categorical variables and median (IQR) and mean ± SD for numerical variables. A p-value of less than 0.05 was considered statistically significant. A Chisquare test was used to assess the relationship between ARB and ACEIs users with NT-proBNP levels at admission and at 1-month follow-up, illustrated by a Box and Whiskey plot. A Kaplan-Meier curve was used to estimate the survival time (Table 2 and Figure 4).
| Variable | N (%) |
|---|---|
| ACEIs | 52 (33.5) |
| ARBs | 57 (36.8) |
| NO ARBs/ACEIs | 46 (29.7) |
| GDMT | 82 (52.9) |
Note: ACEIs=Angiotensin Converting Enzyme Inhibitors; ARBs=Angiotensin Receptor Blockers; GDMT= Guideline Directed Medical Therapy.
Table 2: Baseline pharmacological characteristics, N=155.
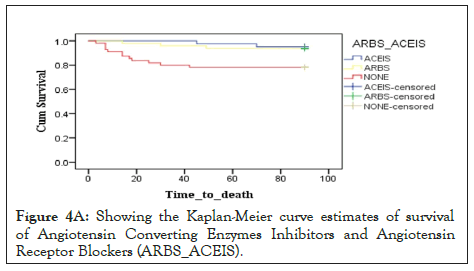
Figure 4a: Showing the Kaplan-Meier curve estimates of survival of Angiotensin Converting Enzymes Inhibitors and Angiotensin Receptor Blockers (ARBS_ACEIS).
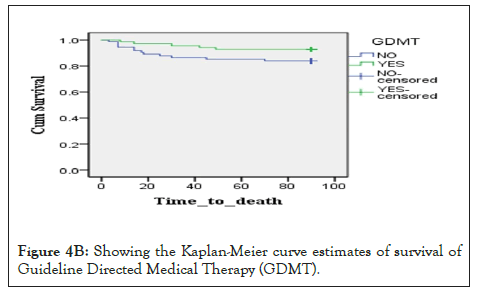
Figure 4b: Showing the Kaplan-Meier curve estimates of survival of Guideline Directed Medical Therapy (GDMT).
Ethics aspect
Ethical clearance to conduct the study was obtained from Muhimbili University of Health and Allied Sciences’ Ethical Review Board. Permission to do the study was obtained from JKCI management. Informed consent was obtained from all study participants before they were enrolled in the study. Cases eligible to participate in the study were included only after being provided with informed consent.
Patient baseline demographic and clinical characteristics are reported in Table 1. The mean age of the study patients was 48 ± 16 (range 18-81) years, and 81 (52.3%) of the patients were males. Most study patients were living in Dar es Salaam 108 (69.7%), were married 112 (72.3%) and were college graduates 71 (45.8%). Cigarette smoking and alcohol consumption was present in 19 (10.3%) and 29 (18.7%) of study patients, respectively. Hypertension was present in half of the study patients 78 (50.3%), while history of diabetes mellitus was present in 31 (20%) study patients. The mean systolic and diastolic blood pressure was 124 mmHg ± 20 mmHg and 76 mmHg ± 13 mmHg respectively. The mean BMI among study patients was 22.6 kg/m2 ± 6.3 kg/m2. Majority of the patient studied had reduced LVEF with a mean of 37.3% ± 10.7% (Table 3 and Figure 5).
| - | ARBs | ACEIs |
|---|---|---|
| AGE, years (mean) | 51 | 46 |
| Male (mean) | 24 | 20 |
| SMOKING (n) | 47 | 39 |
Note: ACEIs=Angiotensin Converting Enzyme Inhibitors; ARBs=Angiotensin Receptor Blockers.
Table 3: The below mentioned table showing the distribution of confounder’s.
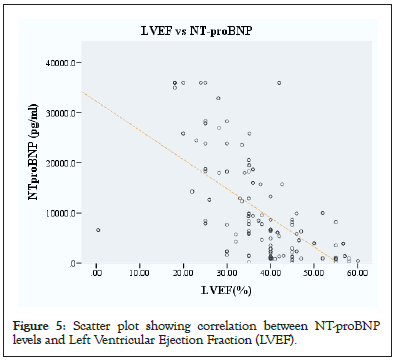
Figure 5: Scatter plot showing correlation between NT-proBNP levels and Left Ventricular Ejection Fraction (LVEF).
Heart failure is known to be a progressive disease, and patients are at higher risks of suffering cardiovascular events at any point of their disease course, despite of the advances of newer drugs and procedures [9]. Increased prevalence of HF in Tanzania, has made a need to search for regimens to improve the quality of life of these patients and in order to do so, ACEIs and ARBs have been among the groups of drugs recommended to improve the process of cardiac remodeling and improve quality of life [10]. Different studies have shown the effectiveness of ACEIs over ARBs, although in Tanzania no such studies have been conducted, furthermore, no studies have been conducted using natriuretic peptides (NT-proBNP) to compare the two group of drugs.
Majority of the patients in Tanzania who are having heart failure are middle aged, as seen in this study, the median age is 48years, with risks like hypertension (50.3%), rheumatic heart disease (31.6%) and diabetes mellitus (20%) [11]. Among the risk factors for the latter diseases includes poverty which mainly lead to life style changes. These factors make it difficult for majority of the patients to be prescribe a GDMT compared to western countries [12]. To be on a GDMT, at least a patient should be on either ACEIs/ARB or ARNI with a betablocker, Mineralocorticoid Antagonist Receptor (MRA) and a Sodium Glucose Cotransporter 2 (SGLT2) Inhibitor [13]. This has proved to be a challenge in Tanzania, with only 52.9% receiving a GDMT accounting to almost half of the patients not being on GDMT for heart failure. Patient under GDMT in this study were on a loop diuretic, ACEI or ARB, MRA and beta blocker once out of pulmonary edema. Our study has also shown that 33.5% and 36.8% of patients had been on ACEIs and ARBs respectively. Enalapril 5 mg, twice daily and lisinopril, 10 mg, once daily dose was the two ACEIs used within the patients studied, while Candesartan, 8mg, once daily and Telmisartan, 80 mg, once daily doses were the ARBs used.
There is evidence that Natriuretic Peptides are independent predictors of total mortality, cardiovascular mortality, and HF hospitalizations in both acute and chronic HF patients [14]. In hospital BNP changes with HF patients appear to be a strong independent predictor of re-hospitalization and mortality [15]. In this study, patient on ACEIs, not only had, lower levels of NT-proBNPs on admission, but also at 1 month follow, there was a significant drop of the levels comparing with those who were using ARBs. These biomarkers can predict mortality as published in the previous study done with the same cohort. The survival curves in this study did not show any statistical difference between the two groups (ACEIs and ARBs groups), though using the biomarkers, it still suggests that ACEIs patients will benefit more in terms of the primary outcome. ARNIs have been a newer group introduced, which has showed to be effective than ACEIs, but due to its less availability especially in remote areas and its high cost, it has not yet been widely used in Tanzania. The limitations of this study are, small sample size and study period, which makes a need and way for a larger study to be conducted.
The limitation of this study is the sample size is small and the follow-up time period was short. This makes a room for a larger study to be conducted. There was minimal bias in the study and the confounding factors were taken into consideration.
Heart failure is rising concern in Tanzania. GDMT has not well been practiced, as shown in this study, with cost of medications being a major contributing factor. ACEIs and ARBs have slightly been cost effective compared to ARNI, and this study shows that ACEIs, as shown from other studies, has more efficacy and overall better clinical outcome than ARBs, but this should be taken under patient-based case, considering the side effects of ACEIs and patients adherence.
The authors declare no financial or non-financial competing interests.
All the authors have contributed to this manuscript in ways that comply with ICMJE authorship criteria. All the authors have read and approved the final version of the manuscript.
The authors acknowledge the cooperation they got from all the patients, and all the members of staff of JKCI who helped this research to run smoothly.
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Mayala MP, Mayala H, Khanbhai K (2023) Comparative Efficacy of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers in Patients with Heart Failure in Tanzania: A Prospective Cohort Study. Acute Chronic Dis. 07:176.
Received: 01-Feb-2023, Manuscript No. ACDR-23-21653; Editor assigned: 03-Feb-2023, Pre QC No. ACDR-23-21653 (PQ); Reviewed: 17-Feb-2023, QC No. ACDR-23-21653; Revised: 24-Feb-2023, Manuscript No. ACDR-23-21653 (R); Published: 03-Mar-2023 , DOI: 10.35248/ACDR.23.07.176
Copyright: © 2023 Mayala MP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.