Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Research Article - (2013) Volume 0, Issue 0
Background: The aim of the present study was to integrate PtcCO2 and PetCO2 models to improve the accuracy and precision of PCO2 measurements.
Methods: 26 ASA physical status I-III postoperative patients recovering from general anesthesia after surgery were selected for investigation. The carbon dioxide partial pressure (PCO2) levels were determined six times during the anesthesia of each patient by using arterial blood gas analysis (PaCO2), end-tidal (PetCO2) and transcutaneous (PtcCO2) measurements. This study tested the hypothesis that combining PetCO2 and PtcCO2 measurements by a regression function, namely Pet_tcCO2, will improve PCO2 measurements during sedation. The Bland-Altman method was used for the statistical analysis, and a p<0.05 was considered statistically significant. Results: A total of 156 data sets (i.e., PaCO2, PetCO2, and PtcCO2) were obtained from 26 patients (63 ± 17 years old; 18 males, 8 females). The mean PaCO2 was 44.3 ± 3.88 mm Hg. The mean PetCO2 was 38.9 ± 4.01 mm Hg. The mean PtcCO2 level was 46.1 ± 3.95 mm Hg. The average PtcCO2-PaCO2 difference was 3.8 ± 1.85 mm Hg. The average PetCO2-PaCO2 difference was -3.9 ± 1.95 mm Hg. When the value of Pet_tcCO2 were combined, the fit equation was Pet_tcCO2=7.24+0.36 × PetCO2+0.46 × PtcCO2, R2= .66, p<0.001. The average Pet_tcCO2-PaCO2 difference was 2.1 ± 1.25 mm Hg.
Conclusions: The combined use of transcutaneous and end-tidal methods could help improve the accuracy and precision of PCO2 measurements. Pet_tcCO2 approach of combining PetCO2 and PtcCO2 techniques could be a new technology for PCO2 measurement in spontaneously breathing patients have no serious lung disease.
Keywords: Arterial blood gas analysis; Anaesthesia; Noninvasive monitoring; End-tidal PCO2; Transcutaneous PCO2
The early detection of hypoventilation in patients helps to prevent hypoxemia. Monitoring the partial pressure of carbon dioxide (PCO2) is an essential means of assessing alveolar ventilation in patients during anesthesia, procedural sedation and emergency care [1-6]. Although the arterial blood gas(ABG) measurement of PCO2 (PaCO2) is regarded as the gold standard technique for PCO2 assessments [7-9], it is an invasive and painful method that requires arterial blood gas analysis. Importantly, PaCO2 also disrupts patient sleep patterns if performed at night.
In general, two different techniques for continuous non-invasive PCO2 monitoring have been introduced into clinical practice, namely, end-tidal partial pressure of carbon dioxide (PetCO2) and transcutaneous partial pressure of carbon dioxide (PtcCO2) measurements [8,9]. These monitoring systems are noninvasive and display real-time data. PetCO2 and respiratory waveforms from a capnograph can provide vital information about CO2 retention and respiratory depression and can serve as an apnea monitor in spontaneously breathing patients [3,4,10]. PtcCO2 sensors are not only widely used in neonatology and in critically ill infants but also used in adult patients during noninvasive mechanical ventilation, the transportation of critically ill adults, bronchoscopy etc [7,11,12].
However, according to the early studies, neither PetCO2 nor PtcCO2 monitoring has provided accurate estimates of PaCO2 [13-15]. PtcCO2 overestimates PaCO2 and PetCO2 significantly underestimates PaCO2. Both the PtcCO2 and PetCO2 provide just an approximate estimation of PaCO2. Clinical use of these monitors cannot be proposed under actual conditions but will be advantageous after correction of the limiting errors.
Therefore, if PetCO2 and PtcCO2 monitoring are determined to be just as accurate as invasive, intermittent PaCO2 measurements, this continuous and non-invasive technique could become the preferred means for monitoring spontaneously breathing patients. The aim of this study was to investigate combining use of the PetCO2 and PtcCO2 methods to offset their deviation with PaCO2. The new model namely Pet_tcCO2 would take advantage of both PetCO2 and PtcCO2 methods to reduce deviations and improve measurement accuracy. The accuracy (i.e., mean difference of the measurements) and precision (i.e., standard deviation (SD) of measurements) of Pet_tcCO2, PetCO2, and PtcCO2 techniques in monitoring PCO2 were evaluated.
This study was approved by the local ethics committee for human research and was performed in accordance with ethical standards outlined in the October 2008 Declaration of Helsinki. Patients were informed of the planned study procedures and were asked for their informed consent. 26 ASA physical status I-III postoperative patients who recovering from general anesthesia after surgery were selected for this prospective clinical-experimental study. Patients with severe cardiovascular or respiratory diseases, such as coronary heart disease, congestive heart failure, or chronic obstructive pulmonary disease were excluded from this study.
A 16-gauge (16-G) IV catheter was placed into the median cubital vein for fluid transfusion, and a 20-G arterial catheter was inserted into the left radial artery under local anesthesia for ABG sampling. The catheters were flushed using a pressure bag with 500 mL of heparinized saline. Before anesthesia, patients’ heart rate (HR) and arterial blood pressure were recorded as the preinduction values. Anesthesia was induced with propofol (4 μg/mL using the target concentration infusion), fentanyl ( 2 μg/kg), and atracurium (0.6 mg/kg).
After surgery, patients were kept in the postanesthesia care unit where routine monitoring consisted of electrocardiogram and noninvasive arterial blood pressure. Patients’ lungs were ventilated with 100% oxygen (2 L/min), and their tidal volume and respiratory rate were adjusted to maintain the PetCO2 within 35 to 45 mm Hg. Anesthesia was maintained with propofol (2 g/mL with target concentration infusion model), desflurane (6%), and fentanyl to keep the blood pressure and HR within 20% of the preinduction values. Additional analgesia was provided by IV paracetamol and morphine as required. The study period started with patients’ breathing spontaneously and receiving oxygen (3L/m-1) through a facemask.
PetCO2 was calculated by a portable capnometer (Tidal Wave Sp, Model 715, Novametrix Medical System Inc.) from a facemask. The device had a 5-Hz internal sampling rate and identified the highest PCO2 level during the last third of expiration by using a built-in proprietary algorithm.
To monitor PtcCO2, a TINA TCM4 device (Radiometer Copenhagen, Brønshøj, Denmark) was used. The TCM4 device provides PtcCO2 information continuously and noninvasively. Before the sensor being placed on patients’ ear lobes, the electrode membrane and skin at the sensor site were cleaned with alcohol, and the electrode was calibrated. The working temperature of the electrode was kept between 41 and 42°C.
A stationary ABL625 blood gas analyzer (Radiometer Copenhagen Brønshøj, Denmark) was used to determine arterial CO2. ABG samplings were intermittently obtained for every patient at six time points (i.e., 15, 30, 45, 60, 90, and 120 min). The measurement started after the transcutaneous sensor had been on the patients’ ear lobes for 15 minutes and the patients’ blood pressure, HR, tidal volume, and respiratory rate were constant to obtain the stable PtcCO2 and PetCO2. PetCO2 and PtcCO2 were simultaneously and continuously monitored, and values were recorded at 1-min intervals before each arterial blood sample was taken.
Previous studies [5-7] showed that PetCO2 and PtcCO2 correlate well with PaCO2. Most time PtcCO2 values are higher than PaCO2 and PetCO2 values are lower than PaCO2. We supposed the new Pet_tcCO2 method could combine PetCO2 and PtcCO2 techniques to offset the deviation. Thus, this study integrated PetCO2 and PtcCO2 methods into a single approach, namely Pet_tcCO2, and its accuracy was compared with that of PaCO2 techniques. Because PetCO2 and PtcCO2 are linear correlation with PaCO2, the combined value Pet_PtcCO2 could be also linear correlation with PaCO2. PaCO2 was the dependent variable, while PetCO2 and PtcCO2 values were considered arguments. Experimental data were used to calculate the coefficient of the linear model.
Statistical analysis was performed with Statistical Product and Service Solutions (SPSS) software version 18. Linear relationships alone (i.e., correlations and correlation coefficients) do not adequately demonstrate the consistency between two clinical measurement techniques [16-18]. Bland-Altman analysis [19] was applied to assess the mean bias and limits of agreement (LOA) (± 2 standard deviation of bias) of PaCO2 and PtcCO2, PaCO2 and PetCO2, PaCO2 and Pet_ tcCO2. According to the quality criteria for blood gas analyzer devices, variation in the mean bias of PtcCO2 and PetCO2 and PaCO2 of 3.5% and a variation of LOA of 12.5% could be accepted. A mean bias ≤ ± 2.5 mm Hg and LOA ≤ ± 5 mm Hg was defined as good agreement. Differences were considered statistically significant when p<0.05.
Mahalanobis distance [20] was used to compare the similarity of PCO2 measurements by PaCO2, PtcCO2, PetCO2 and Pet_tcCO2. The smaller Mahalanobis distance of the two methods means the more similarity of the two methods.
The following equation is defined to calculate the deviation of the different methods.
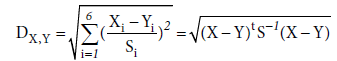 (1)
(1)
Note that X=PaCO2 and Y∈{PetCO2, PtcCO2, Pet_tcCO2}. S is a covariance matrix. The variable i represents the time point of the measurements. And DX,Y represents the Mahalanobis distance between the measure method of X and Y.
A total of 156 data sets (i.e., PaCO2 (immediate response mobile analyzer (IRMA)), PetCO2, and PtcCO2) from 26 patients (18 males, 8 females) were obtained after receiving informed consent. Patients were 63 ± 17 years old (range, 31-81 years) and had body mass indices of 28.0 ± 7.9 kg.m-2.
All data sets (n=156) were complete, and the different measurement techniques were performed without complications. Table 1 shows the results of PaCO2, PtcCO2, and PetCO2 measurements at six time points: 15, 30, 45, 60, 90, 120 min.
| 15 min (mm Hg) |
30 min (mm Hg) |
45 min (mm Hg) |
60 min (mm Hg) |
90 min (mm Hg) |
120 min (mm Hg) |
Total (mm Hg) |
|
|---|---|---|---|---|---|---|---|
| PaCO2 | 40.5 ± 3.76 | 42.9 ± 4.72 | 45.8 ± 2.76 | 46.2 ± 3.44 | 43.5 ± 2.94 | 41.6 ± 1.99 | 44.3 ± 3.88 |
| PetCO2 | 36.3 ± 4.13 | 38.8 ± 3.94 | 39.2 ± 2.84 | 39.6 ± 3.91 | 37.9 ± 5.11 | 36.9 ± 4.23 | 38.9 ± 4.01 |
| PtcCO2 | 39.7 ± 3.62 | 44.1 ± 4.34 | 47.5 ± 5.37 | 47.8 ± 3.54 | 46.7 ± 2.35 | 45.1 ± 3.82 | 46.1 ± 3.95 |
Table 1: Comparison of PCO2 values determined using the various methods.
In these samples, both PtcCO2 and PetCO2 were correlated with PaCO2. As shown in Figure 1A and Figure 2A, the linear regression equations between PtcCO2 and PetCO2 and PaCO2 were obtained (PaCO2=0.63×PtcCO2+12.89, r2=0.53, p<0.01; PaCO2=0.59×PetCO2+19.35, r2=0.45, p<0.05). In addition, PtcCO2 was also correlated with PaCO2 at each time point (r2=0.59, 0.65, 0.51, 0.61, 0.66 and 0.54, respectively, p<0.05). And PetCO2 was also correlated with PaCO2 at each time point too (r2=0.47, 0.44, 0.53, 0.65, 0.57 and 0.56, respectively, p<0.05).
Figure 1: (A) Correlation between PaCO2 and PtcCO2 shows a coefficient r=0.73. Regression analysis: PaCO2=0.63×PtcCO2+ 12.89, r=0.73, p<0.01. (B) Bias and LOA between PaCO2 and simultaneously measured PtcCO2 measurements. The solid line represents the mean of the differences (= bias) between PaCO2 and PtcCO2 (3.8±1.85 mmHg). The dashed lines are equivalent to 2SDs of the means (= LOA).
Figure 2: (A) Correlation between PaCO2 and PetCO2 shows a coefficient R2=0.84. Regression analysis: PaCO2 =0.51 × PetCO2+19.35. (B) Bias and LOA between PaCO2 and simultaneously measured PetCO2 measurements. The solid line represents the mean of the differences (= bias) between PaCO2 and PetCO2 (–3.9 mmHg). The dashed lines are equivalent to 2SDs of the means (= LOA).
Using a Bland-Altman analysis to determine LOAs (i.e., mean ± 2SD), the three techniques showed different accuracies (Figures 1B and 2B). The bias and precision of transcutaneous measurements (3.8 ± 1.85 mm Hg, not significant (n.s.)) were comparable and revealed no significant differences. The bias and precision of endtidal measurements (-3.9 ± 1.95 mm Hg, n.s.) were comparable and revealed no significant differences too. Results showed a linear relationship between PetCO2 and PaCO2, and the PetCO2 method seemed to underestimate PaCO2 values. The PetCO2 technique resulted in 6 outliers with a negative bias. Therefore, this technique was below the given interval (mean ± 2 SD), which causes an underestimation of actual PCO2 values determined by reference measurements. An analysis of the transcutaneous measurement revealed 10 outliers above the given interval with a positive bias. This finding suggests that the PtcCO2 technique systematically overestimated PCO2 levels.
The previous results showed that PetCO2 and PtcCO2 values were significantly correlated with the PaCO2. SPSS was used to calculate multiple linear regression equation Parameters. The linear regression module was selected. PetCO2 and PtcCO2 acted as the independent variables, and PaCO2.acted as the dependent variable. A best-fit formula (Pet_tcCO2=7.24+0.36×PetCO2+0.46×PtcCO2, R2=0.66, p<0.01; Figure 3) and curve were obtained. Pet_tcCO2 value was obtained from the formula. The difference between Pet_tcCO2 and PaCO2 was shown in Figure 4. The bias and precision of Pet_tcCO2 measurement (2.1 ± 1.25 mm Hg, n.s.) were comparable and revealed no significant differences to PaCO2.
Figure 3: Pet_tcCO2 matched curve of PtcCO2 and PetCO2 was added to Average course of PaCO2 and PtcCO2 and PetCO2 measurements during sedation. Arterial samples were drawn at six time points: 15, 30, 45, 60, 90, 120 min after the start of data recording; PtcCO2 and PetCO2 were measured continuously.
Figure 4: Correlation between PaCO2 and Pet_tcCO2 shows a coefficient R2=0.66. Regression analysis: PaCO2 = 0.83 × Pet_tcCO2+5.47. (B) Bias and LOA between PaCO2 and simultaneously measured Pet_tcCO2 measurements. The solid line represents the mean of the differences (= bias) between PaCO2 and PetCO2 (2.0 mmHg). The dashed lines are equivalent to 2SDs of the means (= LOA).
In order to compare PtcCO2 and PetCO2 and Pet_tcCO2 which had the smallest deviation to the PaCO2, Mahalanobis distances from PaCO2 to PtcCO2 and PetCO2 and Pet_tcCO2 were calculated through the experiment data. The result was showed in Figure 4. The Mahalanobis distance between PetCO2 and PaCO2 was 83.4, PtcCO2 and PaCO2 being 51.3, Pet_tcCO2 and PaCO2 being 21.6, respectively. Pet_tcCO2 was found to be more similar to PetCO2 and PtcCO2 methods.
The purpose of this study was to combine the use of PetCO2 and PtcCO2 to improve the acquisition and reliability of PCO2 measurements of spontaneously-breathing patients after general anesthesia. Some of the earlier studies have confused the role of capnography or transcutaneous in predicting PaCO2. Russel et al. [21] found significant correlation between capnography and arterial blood gas measurements. Belpomme et al. [22] reported that wide variations in gradients exist between PaCO2 and PetCO2 values that depend on patient conditions. They concluded that the PetCO2 technique is not useful in pre-hospital ventilation management (bias up to 8.6 mm Hg). On the contrary, Yanagidate et al. [10] concluded capnograph can provide vital information about CO2 retention and respiratory depression and can serve as an apnea monitor in spontaneously breathing patients. Some research reported that transcutaneous PtcCO2 monitoring can continuously and reliably measure PaCO2 in pediatric and geriatric patients during surgery [8,23,24]. But some research found the results from transcutaneous PtcCO2 have been shown to be delayed [7,8]. Furthermore, some studies were controversial in terms of the reliability of PtcCO2 [7,25,26]. Therefore the relationships and differences between PetCO2 and PtcCO2 methods and PaCO2 method should be made clear.
Our study compared PetCO2 and PtcCO2 methods with PaCO2 method. Results showed that measurements by transcutaneous methods on patients undergoing general anesthesia were in good agreement with PaCO2 measurements (3.8 ± 1.85 mm Hg, n.s.). The PetCO2 method also had acceptable results (-3.9 ± 1.95 mm Hg, n.s.). We found the trend PtcCO2> PaCO2>PetCO2 from the result. It was the overall trend, which was based upon means of PCO2 at each time point. Although some individual trend differed from the trend listed above. We found that PtcCO2 was over estimated compared with PCO2 and PetCO2 was underestimated compared with PCO2 most of the time. PtcCO2 measurements were almost all in the positive direction beside PaCO2 while PetCO2 were in the negative direction. So we supposed that combining these two methods measured value would offset the error between them. This is the original idea when we carried out the experiment. We synthesized PetCO2 and PaCO2 measurements into a linear regression model and obtained the equation Pet_tcCO2=7.24+0 .36×PetCO2+0.46×PtcCO2, R2=0.66, p<0.01.
The experiment result showed the trend that PtcCO2 was higher than PaCO2. The main reason was that the sensor temperature improved local perfusion (capillary arterialization) and increased local production of carbon dioxide in the tissue, which leads to falsely high measurements [14]. On the contrary, PetCO2 measurement was always underestimated compared with PCO2 because gas from lung units that were ventilated but not perfused (alveolar dead space or high ventilation-perfusion ratio units) contains little or no carbon dioxide. When this gas was mixed with the gas from “normal” lung units, the resultant concentration of carbon dioxide was reduced in the expired gas. This phenomenon accounts for the fact that PetCO2 was lower than PaCO2 [27]. Many authors have reported the relationship between PetCO2 and PaCO2 during anesthesia. Nunn and Hill [28] reported that mean PaCO2 exceeded mean PetCO2 by 4.5 to 4.7 mm Hg during anesthesia, with either controlled or spontaneous respiration.
Most investigators focused their reports on the accuracy and precision of PtcCO2 or PetCO2, whereas the reliability of them were not or less discussed. We combined used of PtcCO2 and PetCO2 these two methods and this can not only help to improve the accuracy and precision but also help to improve the reliability. Bland-Altman analysis was used in the present study to measure the agreement between Pet_ tcCO2 and PaCO2. Previous studies showed that a difference of 5 mm Hg or less between PaCO2 and other noninvasive measurements was a clinically acceptable difference [19,29,30]. The mean of Pet_tcCO2 sub PaCO2 difference was 2 mm Hg, which indicated that there was no difference between Pet_tcCO2 and PaCO2. The Mahalanobis distance between Pet_tcCO2 and PaCO2 was 21.6 which was smaller than PtcCO2 and PetCO2, which meant Pet_tcCO2 was more similar to PtcCO2 and PetCO2. The scatter diagram of Pet_tcCO2 and PaCO2 showed that 7 points were outside of the acceptable range. While the scatter diagram of PetCO2 and PaCO2 showed that 6 points were outside of the acceptable range. In fact, no new equipment was used to measure the PCO2 so that the precision could not be improved by this method.
The combined Pet_tcCO2 technology can be easily applied to Clinical application. Pet_tcCO2 model could be constructed as software embedded into the original anesthesia machine or ventilator equipment. Most of the existing monitoring devices have a digital output interface. PetCO2 and PtcCO2 data can be easily collected and put into the processing equipment such as the anesthesia machine or ventilator equipment. PetCO2 and PtcCO2 act as the input variables, clinicians can see the Pet_tcCO2 value shown from the software. Because the measure methods of PtcCO2 by transcutaneous and PetCO2 by endtidal carbon dioxide are monitored normally, no additional cost will be needed to carry out the new approach.
Although this combine method could improve higher accuracy for the measurement of PCO2 in general anaesthesia on adult patients, the two different devices need to be used on the patients. For those who do not need these devices for real time monitor, the costs will be increased. But for the serious patients who want to have a ideal way to monitor PCO2 and release pain, this technology is acceptable.
When preparing to use this new method of measuring PCO2, it is important to consider the patients’ basic health condition. Care should be taken to ensure that the patients’ face mask should not leak gas. Transcutaneous capnometry has already been established in clinical applications. In order to make the combine module simple, findings from this study were based on patients with stable cardiac statuses, stable body temperatures, no lung disease, and normal capnograms. Thus, further experiment should be carried out on patients with severe respiratory failure to be monitored using this approach. The sample size of this study was relatively small. Comparisons of complication rates for the two different monitoring techniques will require larger patient numbers because complications in patients undergoing procedural sedations were rare in the emergency department studied. Thus, this study would need to be conducted with a larger sample size to prove its results. With small data sets, the use of artificial intelligent technology, such as support vector machines, can be used to train and obtain model parameters that realize individuation monitors and improve accuracy.
The combined use of transcutaneous and end-tidal methods could help improve the accuracy and precision of PCO2 measurements. Results of this study demonstrate that the Pet_tcCO2 approach of combining PetCO2 and PtcCO2 techniques is a new technology for PCO2 measurement in general anaesthesia on adults patients who have no serious lung disease.