Research Article - (2022)Volume 6, Issue 5
Objectives: The aim of this study was to assess the potential of a smear-layer removing agent (citric acid) vs an accepted gutta-percha-softening agent (xylol) as an alternative substance for removing the root canal filling materials, while investigating the potential for associated demineralization effects.
Materials and methods: Seventy healthy, recently extracted premolars were used, ten as control, with sixty with their canals enlarged, shaped and cleaned and obturated using lateral compaction. Teeth were distributed into 3 groups as follows: 1) no solvent and mechanical removal of the filling materials; 2) 1 ml of Xylol for 1 minute followed by mechanical removal; and 3) 10% citric acid for 1 minute followed by mechanical removal. Two sections of the root were used, one for Raman spectroscopy analysis to evaluate morphological changes in dentine surface and the other for micro-hardness testing (Vickers).
Results: The use of 10% citric acid in in the removal of gutta-percha and sealer was more effective than the xylol and mechanical group (p<0.05), presented less remnants of filling material debris and with non-observable demineralizing effects.
Clinical relevance: This research showed how citric acid can be used as an alternative in endodontic retreatment, how efficiently removed the filling material without damaging the dental tissue.
Conclusion: Citric acid might be considered as a viable alternative in the removal of gutta-percha and sealer during root canal retreatment.
Citric acid; Dentine demineralization; Raman spectroscopy; Root canal retreatment; Xylene
Options to retain teeth for which previous root canal procedure have been unsuccessful are limited to either nonsurgical (retreatment) or surgical revision. Nonsurgical management relies on thorough debridement of the root canal system [1]. Generally, this procedure is less invasive and reflects good outcomes [2,3]. Several techniques have been proposed to remove filling materials from root canal system, including the use of hand k and hedströem files, nickel-titanium rotary instruments both regular and retreatment, gates glidden burs, heated instruments, ultrasonic tips, and lasers. Often many of these techniques included the application of small amounts of solvents to dissolve the remaining materials after mechanical removal.
During the revision procedures, irrigants are used to help to improve debridement by rinsing out debris, dissolving tissue, and disinfecting the root canal system [4,5]. In this process, complete removal of guttapercha and sealer from root canal walls can be time consuming and the apical extrusion of debris may lead to apical irritation and flare ups. While a vast array of organic solvents such as, xylol, orange oil, eucalyptol, tetrachloroethylene, chloroform, halothane and irrigant solutions, like Ethyl-Diamine-Tetra-Acetic Acid (EDTA), sodium hypochlorite (NaClO) and citric acid (C6H8O7) have been used for this purpose, all may contribute to post treatment problems if extruded in great amounts.
Xylol is one of the organic solvents commonly used for the removal of root filling materials due to its capacity to dissolve both, guttapercha and sealer [6-9]. However, several authors and governmental health agencies have reported toxic effects on tissues with xylol, such as mucosal irritation, alterations at the central nervous system and even death caused by breathing on humans exposes to xylol [10-12]. To minimize conflicts between effectiveness and cytotoxicity, other substances (i.e., eucalyptol, orange oil) have been used without harmful effects but resulting in less effective outcomes [13,14]. Likewise, chloroform dissolves gutta-percha more efficiently than any other tested solvent, but it may also react chemically with a significant decrease in enamel and dentine micro hardness. Therefore, while chloroform and xylol are the most efficient solvents, they are also the most toxic used in this revision procedure [15]. Thus, a suitable solvent able to remove gutta-percha and sealer satisfactorily, without modifying physical, chemical and mechanical properties of the dentine is needed. Based on this perspective, citric acid, which is less harmful to the human body, and can also be used as a root canal irrigant [16], may serve to assist in the removal of previous root canal fillings. Studies performed by optical and scanning electron microscopy on dentinal walls have shown that the use of a 50% volume citric acid cleanser in root canals after pulpectomy, provides well-cleaned surfaces leading to improved penetration of resin into the dentinal tubules and better wall adaptation of gutta-percha, without any contraindications in clinical practice [17]. Compared to EDTA, citric acid has a higher chelating action and better capacity to remove the smear layer, which implies the possibility of being used in the revision process in place of xylol [18].
According to Pashley et al., the smear layer contains an organic phase, consisting of collagen residues and glycosaminoglycans stemming from pulp cells, and an inorganic phase of organo-mineral content composed of two distinct superimposed layers [19]. The first inorganic layer is easy to remove from the canal wall, whilst the second layer occludes the dentinal tubules and is steadily adhered to the canal walls. Solutions, such as citric acid might be able to remove obturation materials, by dissolving inorganic constituents of canal walls and also by changing the solubility and permeability of hydroxyapatite-rich peritubular, which in turns increases the size of dentinal tubules [20]. However, the chelating action of citric acid of the dentinal tubules suggests a different protocol for retreatment. Instead of dissolving the filling materials as solvents do the combination of chelating action and capability of citric acid to impact sealers could break the interface gutta-percha/sealer/dentine, providing an effective alternative way to remove residual materials [21,22]. This becomes even more relevant when considering the possible formation of xylopercha (reaction between xylol and gutta-percha), a material that is very difficult to remove from dentinal walls [23,24].
There exists an ongoing debate regarding the use of citric acid in teeth due to possible dentine demineralization. Some authors have established a protocol using citric acid as irrigant solution, based on reports concluding its safety in terms of fracture resistance at a concentration of 50% (V/V) for prolonged periods of time [25]. However, other studies have found dentine demineralization when exposed to a 30% (V/V) citric acid for 2 minutes in water, and even reduced surface hardness of enamel [26,27]. Considering these previously reported results, the present study aimed to evaluate the use of 10% (V/V) citric acid solution as a substance to assist in the removal of old root canal fillings. In pursuing this aim, the cleaning effectiveness and possible demineralization effects of citric acid were compared to that exhibited by xylol through Raman spectroscopy and Vickers micro hardness tests.
The null hypothesis tested was that citric acid has the same capacity in removing obturator material and alteration in dentine hardness as xylol.
Teeth selection
Seventy healthy premolars extracted for orthodontic reasons from patients under 25 years-old with complete apical formation and mild radicular curvature (0° to 15°) were used [28]. Teeth with calcified canals and apical or coronal fractures were excluded. Teeth were decoronated 2 millimeters at the cement enamel junction and kept in a 10% formalin saline solution.
Root canal procedures
Canal preparation: Canal patency using #10 K-type file (Maillefer, Ballaigues, Switzerland) was established in 60 teeth, with the working length -0.5 mm of major foramen. Single file, Wave One Gold® Primary (0.25 mm/.07 Dentsply/Sirona Ballaigues, Switzerland) was used for cleaning and shaping using a X-smart plus® motor (Dentsply/ Sirona Ballaigues, Switzerland); done by thirds (cervical, middle and apical), using 15 ml of 5.25% sodium hypochlorite (NaOCl) as irrigant (Enzohip-5 Eufar®, Medellin, Colombia). One ml of Ethyl- Diamine-Tetra-Acetic Acid solution (EDTA) 17% (Eufar®, Medellin, Colombia) was used as final irrigant for one minute and deactivated with 2 ml of saline solution.
Canal obturation: All canals were filled using lateral compaction; #35 standard gutta-percha as master cone (Maillefer, Ballaigues;Switzerland), resin-epoxy based sealer (Topseal® (Dentsply/Sirona, Ballaigues, Switzerland) and standard #20 and #15 accessory cones (Maillefer, Ballaigues;Switzerland) with an A25 spreader (Dentsply/Sirona, Ballaigues, Switzerland). The coronal gutta-percha was removed 2 mm below the cement-enamel junction, followed with vertical compaction using a B60 plugger (Dentsply/Sirona, Ballaigues, Switzerland). Coronal access was sealed with Vitrebond® (3M ESPE). The quality of obturation phase was checked using periapical radiographs. Subsequently, all teeth were stored for two months in sterile jars under 95% humidity and 37°C conditions.
Control groups: Two control groups were used: i) Control group 1 (negative control): 5 randomly chosen teeth without any treatment; and ii) Control group 2 (positive control): 5 teeth with the pulp mechanically removed and subsequently irrigated with NaOCl 5.25%, 17% EDTA and saline solution.
Canal retreatment: The coronal seal was removed using a #2 diamond round sterile bur and a #1 Peeso reamer (Maillefer, Ballaigues, Switzerland) was used to remove root coronal filling material. The specimens were divided into 3 groups (20 teeth each) for testing the substances, as follows:
i) Group 1: Mechanical retreatment without any solvent.
ii) Group 2: 1 ml of Xylol (Enzohip-5 Eufar®, Medellin, Colombia) for
1 minute followed by mechanical retreatment.
iii) Group 3: 1 ml of a 10% citric acid for 1 minute followed by
mechanical retreatment.
Due to the chelating action of citric acid and concentration, one minute was used for a complete elimination of the smear layer and to prevent erosion of the calcium-rich peritubular dentine. Additionally, all samples were irrigated with 15 ml of 5% of sodium hypochlorite during the procedure (Enzohip-5, Eufar®, Medellin, Colombia) to rinse debris and 1 ml saline solution to neutralize citric acid [29].
Procedure for sample analysis
All samples, including controls, were split along the longitudinal axis to the root. Buccal and lingual sections were used for Raman spectroscopy analysis and micro-hardness tests (Vickers), respectively.
Raman spectroscopy
Raman measurements were performed by an ocean optics IDR-Micro 785 spectrometer with 15 seconds dwell time (10 scans). A Laser line of 785 nm wave length with optical power of 10.0 mw and 4.0 mw was applied to measure wall dentine and canal dentine, respectively. Raman spectra for the different radicular dentine thirds (cervical, middle and apical) were taken and their intensity was normalized to unity. No significant differences were found between the spectra when compared with previous reports (Figure 1). Therefore, dentine spectra for every tooth were recorded as reference.
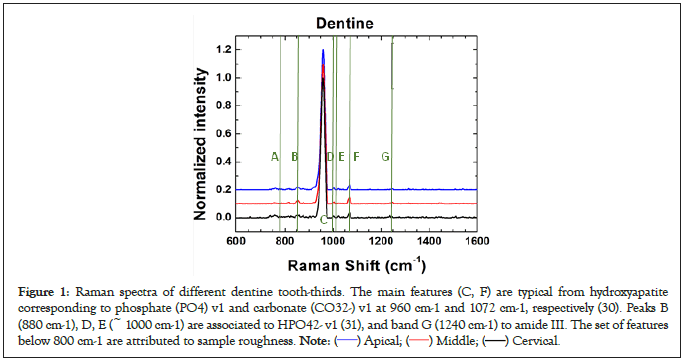
Figure 1: Raman spectra of different dentine tooth-thirds. The main features (C, F) are typical from hydroxyapatite
corresponding to phosphate (PO4) v1 and carbonate (CO32-) v1 at 960 cm-1 and 1072 cm-1, respectively (30). Peaks B
(880 cm-1), D, E (~ 1000 cm-1) are associated to HPO42- v1 (31), and band G (1240 cm-1) to amide III. The set of features
below 800 cm-1 are attributed to sample roughness. Note: ( ) Apical; (
) Apical; ( ) Middle; (
) Middle; ( ) Cervical.
) Cervical.
Additional reference Raman spectra were taken for gutta-percha and AH-Plus® sealer (Dentsply/Sirona, Ballaigues, Switzerland) (Figure 2a). The uppermost spectrum (Figure 2b), was measured on the middle third section of a radicular canal. Basically, this spectrum can be considered as a superposition of the previously taken reference Raman spectra of dentine (*) plus sealer (+), indicating a canal with sealer. Additional features attributed to surface roughness (**) due to canal concavity are also visible. Clearly, Raman spectroscopy was useful to demonstrate an incomplete removal of filling materials however the goal of the retreatment procedure was not accomplished.
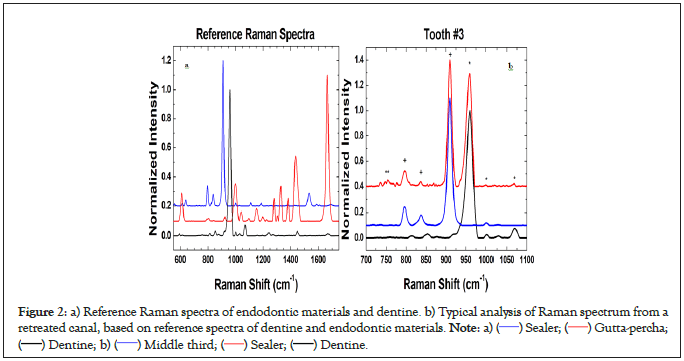
Figure 2: a) Reference Raman spectra of endodontic materials and dentine. b) Typical analysis of Raman spectrum from a
retreated canal, based on reference spectra of dentine and endodontic materials. Note: a) ( ) Sealer; (
) Sealer; ( ) Gutta-percha;
(
) Gutta-percha;
( ) Dentine; b) (
) Dentine; b) ( ) Middle third; (
) Middle third; ( ) Sealer; (
) Sealer; ( ) Dentine.
) Dentine.
Micro-hardness tests
The intrinsic hardness of dentinal structure depends on size, density, orientation and degree of mineral and hydroxyapatite content of microtubules [30-32]. Therefore, demineralization effects due to chemical application could be evaluated through micro-hardness testing, using an indenter probe under a specific load that penetrates a surface during a defined dwell time. The imprinted size or depth left by the indenter, is inversely proportional to surface hardness. The hardness Vickers (HV number) is given by the ratio between the forces applied for the diamond indenter in kilograms-force and the surface area A of the resulting indentation in square millimeters. For a diamond pyramid with 136° angle, HV is given by the relationship (ASTM E384: Standard Test Method for Knoop and Vickers Hardness of Materials).
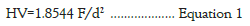
Here, d is the average of the two diagonal lengths of the imprinted diamond pyramid.
To achieve an accurate measurement using the indenter tip, flat surfaces are recommended; extremely rough surfaces reduces data accuracy. Therefore, selected teeth were immersed into acrylic and subsequently polished with #320 and #600 sandpaper until a flat surface was obtained. Indentation was not performed at the canal itself, but at 0.5 mm from the canal edge on a flat dentinal surface.
A Laizhou lyric HVS-1000 micro-hardness tester with a load of 50 g-f (0.49 N) applied to a standard 136° diamond during 20s was used. Micro-hardness is inversely proportional to tubular density, which increases from apical to cervical dentine, different HV values were expected over different tooth regions. Therefore, indentation of cervical, middle and apical thirds was performed for all 70 teeth [33]. This information combined with data from Raman spectroscopy was used to evaluate morphological changes in dentine surface.
Analysis of the information: The normality of the data was determined by the Kolmogorov Smirnov and Shapiro Wilk tests, and the equality of variances using the Levene test. The one-way ANOVA test was performed to analyze the treatments and controls groups independently of where the analysis of the root third was performed, and two-way ANOVA to consider the root third- dependent results. For the Post-Hoc analysis, T-Student and Tukey test were used. In all cases p<0.05 was considered significant. Chi-Square and Fisher's exact tests were used to compare the percentage of material remnant.
Effectiveness of non-sugical treatment revision estimated by raman spectroscopy: Raman spectroscopy was used to compare the efficiency in removing previous root canal filling materials, using two different chemical substances such as xylol and citric acid. First, Raman spectra measured mechanically retreated canals, without any chemical application. The respective Raman spectra in Figure 1 show traces of either gutta-percha or sealer in 50% of the samples.
When comparing xylol and citric acid, 66.7% and 31.6% had filling material, respectively. This result clearly demonstrates higher effectivity in canal cleaning when citric acid was used. There were statistically significant differences at the comparison between the groups. The citric acid group present the lowest percentage of filling material remaining (P<0.05). The xylol group presented the highest percentage (P>0.05) (Figures 3a and 3b).
Figure 3: Raman spectra from canals with different protocols of retreatment: mechanical procedure, xylol and citric acid treatment. a) Samples with residual endodontic filling material. b) Samples free from endodontic filling material.
Demineralization effects: A combination of micro-hardness test and Raman spectroscopy was performed to evaluate the effects of demineralization on the weakness of the root canal structure (dentine).
Micro-hardness test: Teeth irrigated with EDTA (positive control) had basically the same average hardness of those untreated (negative control). EDTA irrigation did not induce changes in micro-tubular dentinal structure. Compared to untreated teeth (positive and negative controls), a slight decrease in average hardness was observed for all applications (mechanical, xylol and citric acid). Despite the noticeable dispersion in the data, the analysis of ANOVA tests indicated that the procedures tested in this study actually influence the results of Vickers hardness. A detailed analysis using the Post-hoc Tukey test found significant differences between the group of xylol retreatment and the other retreatment groups and control groups in all thirds studied. The lowest Vickers hardness values were found in the apical third for all the protocols used compared to other canal thirds (Table 1 and Figure 4).
| Groups/Thirds | Cervical | Middle | Apical |
|---|---|---|---|
| Control 1 | 76.83 ± 16.08 | 83.43 ± 7.94 | 75.35 ± 19.15 |
| Control 2 | 80.75 ± 5.04 | 80.94 ± 4.19 | 72.74 ± 1.44 |
| Xylol retreatment | *66.54 ± 15.88 | *64.62 ± 10.79 | *56.60 ± 16.88 |
| Mechanically retreatment | 73.41 ± 13.97 | 72.63 ± 12.98 | 64.45 ± 17.29 |
| Citric acid retreatment | 76.07 ± 12.85 | 68.16 ± 14.14 | 66.70 ± 13.83 |
Figure 4: Hardness vickers tests performed in differently treated samples, including controls. The reported HV average value includes data measured in the apical, middle and cervical thirds of each specimen. Error bars reflect a considerable standard deviation (~20%), estimated from 60 individual measurements for each group.
Raman spectroscopy
Complementary Raman spectra were taken in order to identify possible demineralization effects on the cervical third of samples with different retreatment methods (Figures 5a-5c). The spectra show the corresponding peaks of dentine with more pronounced bands associated to surface roughness (~700 to 900 cm-1). Dentine Raman spectra did not show significant differences among the different groups. Thus, possible demineralization effects associated to the use of citric acid for retreatment were, at least, not higher than those induced for the use of xylol.
Figure 5: Raman spectra taken on the cervical third of mechanically retreatment (a) xylol-retreatment; (b) 10% citric acid solution retreatment; (c) The selected spectra were recorded on teeth without any trace of filling material.
In the last decade, Raman spectroscopy has gained importance in biomedical field due to the minimal preparation and non-destructive chemical identification for biological samples, even those containing water [34]. Chemical identification is obtained through molecular vibrational modes provided by disperse radiation. In this study, Raman spectroscopy was considered to compare the effectively of substances used in root canal retreatment and also to identify possible dentinal demineralization effects.
In this study, the condition of the sample (a flat surface) required for Raman measurements was not possible because the natural concavity of the canal and roughness of dentin walls; with the anatomical nature of this surface, the Raman signal becomes weak and has a slight impact on the data because the dispersed radiation is strongly absorbed and does not find an easy and direct pathway trough Raman´s detector. Therefore, for an accurate reading the process of Raman spectroscopy was done 10 times for 15 seconds in each specimen, obtaining an average.
Data from the Raman Spectra showed that the use of a 10% citric acid solution was more effective than xylol in removing debris of the remaining filling material. This is due to the fact that Xylol, alters the semisolid-state phase of gutta-percha leading to a paste (xylopercha), which is more difficult to be remove from dentinal tubules. This paste occludes the dentinal tubules, thereby preventing the irrigant solution penetration and desinfection, while the citric acid removed the guttapercha material from canal in very small fragments.
While chemical revision may lead to undesired demineralization effects that might weak the root canal structure, there are no standard protocols related with time, amount and concentration of solvents used, not even their consequences or impact over dentine microhardness due to the possibility of mineral loss; although this is still a matter of debate. Some reports claimed that the use of solvents improved the cleaning efficacy from root canal areas difficult to access with intra-canal instruments [35]. However, there is no solvent that is able to provide a complete removal of filling material and it could be thought that it might have an adverse effect on the bond strength of resin sealer into intra-radicular dentin, in terms of root canal filling or even in fiber posts adhesion [36].
Ideally, mechanical properties like strength, composition and hardness of dentin should not be affected negatively due to irrigation (or any other intervention) procedures or, at minimum any effect should be minimized. The use of EDTA or any acid like citric acid, including sodium hypochlorite causes a progressive dissolution of peritubular and intratubular dentine. This has to be considered as this effect may be magnified when these solutions are applied in the initial procedure prior to revision, which may lead to a possible weakening of dentine, thereby enabling the formation of a crack or fracture. The question is, what is the penetration depth of these solutions used in these applications that would result in sufficient damage inside the tubule; or even if the solutions are not removed during the revision process.
Within the focus of this study, citric acid had to work between the interface material dentine not only over dentine wall as well, in which the presence of gutta-percha and sealer may have impacted on the erosive effect. This outcome differed from that of Turk et al. who reported the smear layer removal and the erosive capacity comparing EDTA, boric acid, citric acid and Desy Clean® (Sojall, Salzburg, Austria) with a sequential use of 2.5% NaOCl on instrumented root canal walls, in which citric acid received the highest erosion scores [37].
Data obtained in this study, within the limitations imposed by the experimental conditions of Raman measurements and microhardness test, showed no significant demineralization effects for any of the specimens in the experimental group. There were no significant differences between the spectra obtained, which correlate with previous reports. Therefore, root canal retreatment had a negative impact on the penetration and bond strength of resin cements to root canal dentine, which may be due to the additional and aggressive activity on the dentine walls during the canal revision procedures. Within this perspective, it is important to use substances that in addition to cleaning do not cause dentine demineralization.
The group treated with 10% V/V citric acid group showed the lowest percentage of residual filler material compared to the Xylol group that presented the highest percentage. EDTA irrigation did not induce changes in the hardness. Teeth with mechanical treatment, xylol and citric acid showed a slight decrease in hardness compared to those without treatment. The use of 10% V/V citric acid in canal retreatment was as effective as Xylol for removing canal filling materials, resulting in a minimal amount of material debris and dentine demineralization. 10% V/V citric acid could be considered as a standard solution for retreatment.
The authors thank Dr. Yuli Berlin-Broner for her concept in the article; Martha Manrique for the Vickers micro-hardness test made at Centro de Tecnología y Automatización Industrial (CTAI) from Industrial Engineering Department of Pontificia Universidad Javeriana in Bogotá (Colombia).
All the authors, declare no conflict of interest.
This study was funded with reosurces from Faculty of Dentistry of Pontificia Universidad Javeriana and the Financial support of Colciencias through Project Scienti 120365843881.
This study was approved by Ethics and Research Committee of the Dentistry Faculty, Pontificia Universidad Javeriana (CIEFOPUJ) from Bogotá-Colombia.
Citation: Méndez-De-La-Espriella C, Rodríguez-Ciodaro A, Moreno-Sarmiento A, Mendieta-Flores D, Yori-Roa D, Gutmann JL, et al. (2022) Citric Acid: An Alternative for the Removal of Root Canal Filling Materials. J Odontol. 6: 630.
Received: 20-Sep-2022, Manuscript No. JOY-22-19279; Editor assigned: 23-Sep-2022, Pre QC No. JOY-22-19279 (PQ); Reviewed: 07-Oct-2022, QC No. JOY-22-19279; Revised: 17-Oct-2022, Manuscript No. JOY-22-19279 (R); Published: 24-Oct-2022 , DOI: 10.35248/JOY.22.6.630
Copyright: © 2022 Méndez-De-La-Espriella C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.