
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2020)Volume 10, Issue 6
This research article focuses on the case report of a 72 years old athletic male from Southwestern Virginia who was chronically exposed to methylmercury via the consumption of tuna and swordfish steaks. The tuna and swordfish fish were wild-caught in the Pacific basin (Viet Nam or Thailand). As a comparison or analyses, Atlantic basin fish samples were obtained as fresh tuna and swordfish steaks from a seafood store in Salem, Virginia USA. Atlantic basin fish were caught off the coast of North Carolina, USA. The patient’s methylmercury toxicity presented clinically as an erythematous rash with macules, patches, papules and petechia on his bilateral ankles. The patient’s blood mercury level was extremely elevated at this time. The patient stopped his daily intake of fish and was observed at an out-patient dermatology clinic over the course of 11 months, during which time blood levels of mercury were repeatedly taken. The patient’s symptoms improved and the blood mercury levels declined. Additionally, Inductively Coupled Plasma with Mass Spectrometry (ICP-MS) analyzed mercury levels from the fish consumed by the patient as well as a group of fish from a different geographical region. While fish and other seafood is frequently noted for its health benefits, it is imperative that the potential toxicities be discussed and researched across the globe, especially in areas in which it is a primary dietary source. There is a great need for local and national public health officials and the various stores and markets where consumers purchase fresh and frozen fish, to provide the public with information on mercury content, and allowable amount and frequency that mercury-laden fish should be consumed, allowing consumers to make informed decisions about which fish to eat.
Mercury toxicity; Methylmercury; Minamata Convention; Swordfish; Tuna; Inductively-coupled plasma/ mass spectrometry
Mercury is considered to be one of the most dangerous heavy metals causing toxicity in humans [1]. It is third on the Agency for Toxic Substance and Disease Registry (ATSD)’s substance priority list, which rates substances based on their risk to human health [2]. Mercury is classified as inorganic or organic [3]. Inorganic mercury includes elemental mercury (HgO), which is also referred to as metallic mercury, mercury vapor, or salts. The organic form of mercury is bonded to a carbon atom, as in ethylmercury (EtHg) and methylmercury (MeHg) [3,4]. In its elemental and methylated forms, mercury is neurotoxic, and in the mercury salt form, it is primarily nephrotoxic [1]. Mercury binds to various biological molecules such as proteins, and hinders their activity. For example, antioxidants such as the reduced/oxidized glutathione system, which supplies 30%-40% of cellular antioxidant capacity, becomes inactive when bound to mercury and loses all antioxidant capacity. Metallothionines can also become bound to mercury, which makes them incapable of binding trace metals such as zinc and copper [1].
This article focuses on an unusual case presentation of methylmercury intoxication. Mercury first enters the environment in the elemental and inorganic forms, primarily released from burned coal or as industrial waste. In the aquatic environment, released inorganic mercury is biotransformed to methylmercury by microorganisms. Aquatic organisms such as small fish and shellfish, consume these microorganisms, which are in turn consumed by larger predators. This movement up the food chain results in biomagnification of mercury in larger predators. Hence larger, longer-lived fish have the highest levels of methylmercury [5]. Methylmercury intoxication, which is the subject of this report, can occur from the consumption of such large, predatory, fish [6,7].
In the United States, open water fisheries are the most common source of fish contaminated with methylmercury [8]. Fish can vary in mercury content based on the species, size, origin, and migratory biology [9]. In fact, a recent study revealed that mercury content of yellowfin tuna is associated with capture location. The largest source of methylmercury contamination in the world is the Pacific Ocean due to the highest global mercury emissions from nearby Asia, Southeast Asia and India. In general, human mercury concentrations are highest in populations that live near coastal areas or have diets high in seafood, specifically in the Arctic, Atlantic, Mediterranean, and Pacific coastal areas [10]. Overall, fish with the highest contamination level are king mackerel, tilefish, tuna, shark, and swordfish [5]. In the United States, tuna is the most frequently consumed source of methylmercury.
Clinical symptoms of mercury toxicity depend on the length and intensity of the exposure [5]. Patients with lower levels of chronic exposure can present with nonspecific health effects like sleep disturbance, fatigue, muscle and joint pain, and gastrointestinal upset. Exposure to mercury, even at low levels, has also been shown to have negative effects on cardiovascular function [11,12]. High exposure to mercury can have detrimental impacts on the central nervous system causing fatigue, irritability, rash, headaches, hearing and cognitive loss, paresthesia in the hands and feet, speech difficulty, incoordination, and in worse cases, death. Pregnant women or women of reproductive age consuming moderate to high levels of mercury in their diet are at higher risk for adverse reproductive health outcomes including stillbirth, low-birth weight, and birth defects [13]. Fetuses exposed to mercury in utero have resulted in significant neurological disorders (e.g., attention-deficit disorder, seizures, mental retardation, and other malformations) and cognitive developmental delays, causing irreversible damage to the central nervous system [14,15].
The toxic effects of mercury vary depending on the form and the dose, duration of exposure, and the life stage of the affected person [4,7]. Methylmercury has a half-life of approximately 70 days, and 90% is excreted in stool (via the bile). About 20% can be excreted in breast milk in pregnant female. Methylmercury deposits in brain, peripheral nerves, bone marrow, kidneys, placenta, and liver causing many different forms of damage to these organ systems [4]. Blood levels of mercury are used to determine both chronic accumulation and recent exposure. The Centers for Disease Control and Prevention define blood mercury levels above 10 μg/L as excessive [5]. The Environmental Protection Agency’s reference level for mercury in hair is less than 1 μg/g (ppm). Urine mercury levels above 35 μg/g creatinine are considered elevated. However, urine measures primarily inorganic mercury exposure and is not usually used to monitor methylmercury since that particular form is excreted in feces [6,8].
For patients with excessive methylmercury levels, like the one presented in this paper, it is recommended to temporarily refrain from eating fish or switch to eating fish low in mercury content in order to decrease exposure. When symptoms have resolved and their blood mercury levels are reduced to less than 5 μg/L, fish may then be slowly added back to the diet. Chelation may be used as an additional treatment modality, but it is generally used for patients showing clinical signs of inorganic mercury toxicity [5].
In this study, we report chronic methylmercury toxicity in a 72 years old athletic male from Southwestern Virginia with a history of ingesting Yellowfin tuna, Bluefin tuna, and swordfish steaks daily for 15 months.
Fish acquisition
All tuna and swordfish consumed by the patient were purchased at a local grocery store in Roanoke, Virginia, USA. Fish were purchased primarily as bulk frozen steaks, 6-8 ounces each, individually wrapped by the importer. The supplier of these fish was Sea Delight, Coral Springs, FL and the fish were wild-caught in the Pacific basin (Viet Nam or Thailand). As a comparison or analyses, Atlantic basin fish samples were obtained as fresh tuna and swordfish steaks from a seafood store in Salem, Virginia USA. Atlantic basin fish were caught off the coast of North Carolina, USA.
Human blood analysis
Blood heavy metals levels, complete blood count (CBC), and complete metabolic panel (CMP) were performed by LabCorp (Burlington, NC) for diagnosis and at intervals subsequent to diagnosis of mercury intoxication. Urine mercury analysis was also performed by LabCorp to rule out inorganic mercury toxicity.
Fish tissue analysis
Mercury levels in fish were determined by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) at the Virginia-Maryland Regional College of Veterinary Medicine, Laboratory for Analytical Research, Virginia Tech, Blacksburg, Virginia. Replicate 0.5-g (wet weight) samples of the fish were weighed directly into modified polytetrafluoroethylene (TFM-PTFE) microwave digestion vessels. The weighed samples then received 3 mL 18.2 MΩ deionized water, 3 mL trace metal grade concentrated nitric acid (70% w/v HNO3), and 1 mL of trace metals grade hydrogen peroxide (30% w/v H2O2). The mixture was briefly swirled, and allowed to sit for 10 minutes before being capped, shaken, and loaded into the microwave digestion vessel carousel. The microwave digestion program ramped from room temperature to 180°C over 20 minutes and held for 10 minutes at that temperature before cooling to 70°C over approximately 10 minutes. Digested samples were clear/colorless with no visible particulates. The digests were quantitatively transferred to 15 mL polypropylene centrifuge tubes, using 2% (w/v) HNO3+0.5% (w/v) HCl to rinse the microwave vessels into the centrifuge tubes for a 10 mL final volume. The concentrated digests were then briefly shaken and vortexed to mix before being diluted for ICP-MS analysis. The digests were diluted 1:10 by adding 0.5 mL of the digests to 4.5 mL of 2% (w/v) HNO3+0.5% (w/v) HCl in fresh 15 mL polypropylene centrifuge tubes. These were again briefly shaken and vortexed to homogenize the dilute solution, and were then loaded onto the auto sampler of the ICP-MS.
Mercury content was quantified using a calibration curve with known concentrations of mercury in the same dilute acid diluent as the samples. ICP-MS analysis of these standards and samples was performed by directly monitoring several mercury isotopes at 199, 200, 201, and 202 m/z. In addition to multiple isotope monitoring, the use of a helium collision cell minimized and/or eliminated potential polyatomic interferences. An on-line addition internal standard solution comprised of bismuth (209 m/z) was utilized to correct for any potential instrumental response variations observed during the analysis. The use of ICP-MS is critical for the analysis due to the higher selectivity and lower detection limits versus those of an ICP-OES type system. Final results are expressed as parts per million (PPM) of mercury.
Patient pre-clinical history
The patient is a 72 years old Caucasian athletic male, pescatarian from Southwestern Virginia and reports that he has not eaten any meat since 1972 but does eat fish. Because of his extensive physical activities including long distance cycling, hiking, kayaking and gym workouts, he acquires his protein by eating eggs, dairy including cheese, whole grains, soy products, beans and nuts. While training for several cross-country bicycle rides, his main source of protein was eggs. He reported eating 4-6 hormone-free, pasture raised chicken eggs every day from February 2016 to May 2017, at which time he developed an allergy to eggs, which presented as asthma- like signs and symptoms including coughing, wheezing, chest tightness and shortness of breath. Beginning June 2017, the patient began eating 6-8 ounces of previously frozen Yellowfin and Bluefin tuna steaks purchased in individual sealed packets (Sea Delight brand) from his local supermarket. About 2-3 times a month he would supplement with a 12-16 ounce swordfish steak. All frozen fish products were caught in Southeast Asia, shipped from either Vietnam or Thailand to Florida, and distributed throughout eastern regions of the United States. The patient reported that he would eat 4-6 ounces of tuna for breakfast, saving 2-3 ounces, which he would eat after work and before each day’s work out. The patient reported that he was aware that wild caught tuna was high in mercury. During January 2019, the patient reported that he was helping an elderly family member blow leaves from his back yard in Cary, North Carolina. Three days later, he noticed a rash with petechiae around his ankles (Figure 1). Eight hours later the rash had progressed up his legs and covered his back and chest. All regions of his body, except for his facial area, were covered in rash.
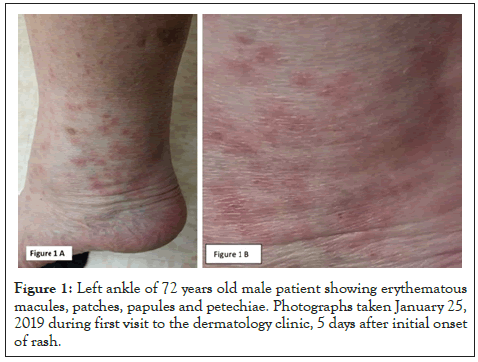
Figure 1: Left ankle of 72 years old male patient showing erythematous macules, patches, papules and petechiae. Photographs taken January 25, 2019 during first visit to the dermatology clinic, 5 days after initial onset of rash.
Patient clinical history
On 1/25/2019 the patient presented to the dermatology clinic with a rash which developed as shown in Figure 1. During this time the rash spread from his legs to his lower back and chest. Patient exhibited no pain, fever or pruritus. Upon examination, he had normal vital signs and displayed erythematous macules, patches and papules scattered on the legs, back and trunk, with a few on the arms. There were no vesicles or signs of secondary infection. Although he had been working outside, no tick or insect bites were noted and patient had no other complaints. Patient reported that he consumed 6-8 ounces of tuna, yellowfin tuna, or swordfish each day, and had been doing so for the last year and was concerned about mercury poisoning from the fish consumption. A CBC, CMP, and heavy metal profile for Hg, Pb, and As were ordered. A 10-day prednisone taper was initiated.
The patients CBC and CMP were normal; however, his blood mercury was extremely high, at 126.2 g/L (Figure 2). Patient had no tremors, muscle weakness, paresthesias, or neurological symptoms, which is extraordinary given the high blood mercury levels. Arsenic and lead were within normal ranges, suggesting the rash had been caused by elevated mercury levels. Poison control was consulted and the patient was advised to refrain from eating any seafood or drinking any well water (patient’s home was serviced well water). To determine the possibility of inorganic Hg intoxication, the levels of Hg in the patients urine were tested, and were within normal range (6 g/24 hr, normal range 0-20), suggesting the offending toxin was methylmercury rather than inorganic mercury. Kidney function was also within the normal range. Following the initial diagnosis, blood heavy metals analysis was repeated on a regular basis, until the patient’s Hg levels returned to normal range, as shown in Figure 2. The time required for this patient’s blood mercury levels to normalize was approximately 10 months. During this time, the patient consumed no fish products.
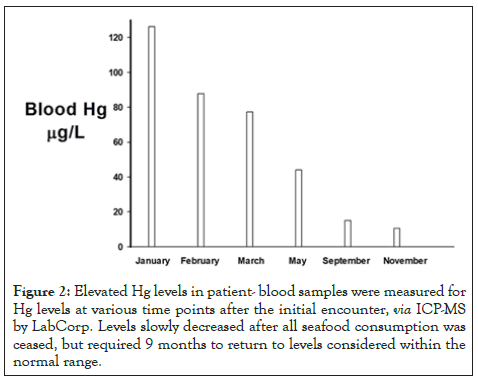
Figure 2: Elevated Hg levels in patient- blood samples were measured for Hg levels at various time points after the initial encounter, via ICP-MS by LabCorp. Levels slowly decreased after all seafood consumption was ceased, but required 9 months to return to levels considered within the normal range.
Next, an attempt to identify the source of the mercury intoxication was performed. The patient’s well water was tested for mercury and other heavy metals, and results were below detection limits (data not shown), suggesting that well water contamination was not the likely culprit. The patient still had frozen samples of the tuna, yellowfin, and swordfish he had been eating. The patient had been purchasing the same brand of frozen fish from a local supermarket for the past year. All fish were imported by Sea Delight LLC (Coral Springs, FL) and were a wild caught product of Viet Nam or Thailand, as per the package labeling. Samples of the fish were analyzed by ICP-MS for total mercury, and the results are shown in Figure 3 (white bars). Total mercury levels in all the samples from fish consumed by the patient were very high, particularly the swordfish. For comparison, the reported USDA levels of mercury in fish are shown in the black bars, (Figure 3). All samples consumed by the patient exceeded the USDA levels reported for fish from the Atlantic basin. We hypothesized that the Pacific basin-caught fish were higher in mercury than Atlantic basin-caught fish. To test this, we compared the fish the patient consumed, to fresh tuna and swordfish caught in the Atlantic basin (North Carolina). Mercury levels in the Atlantic basin fish are shown in the grey bars in Figure 3. The Atlantic basin fish analyzed were within the levels published by the USDA (compare grey to black bars), and were far less than the Pacific-basin fish the patient consumed.
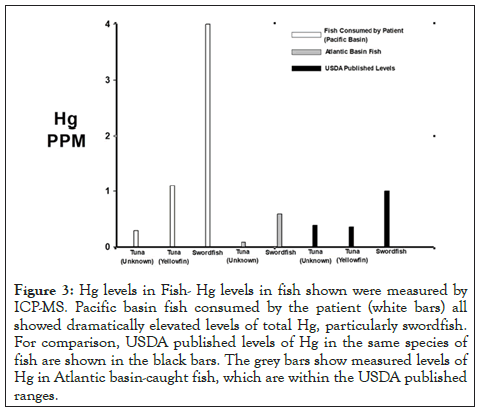
Figure 3: Hg levels in Fish- Hg levels in fish shown were measured by ICP-MS. Pacific basin fish consumed by the patient (white bars) all showed dramatically elevated levels of total Hg, particularly swordfish. For comparison, USDA published levels of Hg in the same species of fish are shown in the black bars. The grey bars show measured levels of Hg in Atlantic basin-caught fish, which are within the USDA published ranges.
Although the patient exceeded the recommended daily intake for tuna and swordfish for quite some time, and would likely have encountered mercury toxicity even if he consumed Atlantic caught fish, the pacific basin fish carried excessively high levels of mercury. We hypothesize that the inordinately high levels of methylmercury in the frozen pacific basin fish consumed by the patient worsened the blood mercury levels even further and could potentially be harmful even at the current recommended consumption levels.
The patient refrained from all seafood consumption over the next year. As shown in Figure 2, his blood mercury levels consistently declined over the next several months. However, it took 9 months to return to within the normal range, consistent with the half life of methylmercury. The rash subsided 16 days after onset. During this time, the patient self-administered 600 mg n-acetyl-cysteine twice each day for 120 days after the diagnosis. Fortunately, the patient exhibited no untoward signs of mercury toxicity, other than the initial rash.
Addressing methylmercury pollution in Pacific basin Fish
In 2013, 128 nations across the world agreed upon the Minamata Convention on Mercury, which aims to reduce the emission rate of mercury into the environment and to reduce its subsequent adverse health effects [16,17]. While these measures have started to improve the current control on mercury pollution, the threat remains to aquatic flora and fauna [18,19]. Unfortunately, this danger is not projected to improve unless further action is taken, leading some authors to support global adherence to the Minamata Convention [10,20-22], which is currently lacking. One of the major driving factors affecting the mercury levels in the oceans, especially in the Pacific basin in areas around Vietnam, Thailand and China, and therefore the wildlife that live in them, is considered to be the environmental emissions due to coal burning and artisanal gold mining activities [23]. We feel that the exceptionally high levels of methylmercury in the patient was due, in part, to his daily intake of tuna and swordfish caught exclusively from this geographic area.
Patient tolerance for high methylmercury blood levels
When ingested, methylmercury binds to glutathione, to make a methylmercury-glutathione complex that is transported throughout the body, crossing the blood brain barrier and placenta with ease [24]. The elimination of methylmercury occurs through the bile, with a fraction of the compound being reabsorbed through the process of enterohepatic circulation, which further slows the elimination of methylmercury to a half-life of ± 70 days [25,26]. This half-life is consistent with the decline in blood mercury shown in our patient. In our patient, other than rash, no other adverse signs were noted. This is remarkable, considered the high methylmercury levels in his blood for an extended period of time (possibly a year prior to diagnosis). Two possibilities arise for this patient’s lack of adverse symptoms other than rash. First, since the patient is an athlete and vegetarian after this diagnosis, his physical health and a diet high in antioxidants may have dampened the effects of the methylmercury. Second the patient may have received some protection from methylmercury toxicity due to his self-administration of 600 mg n-Acetyl-cysteine twice each day. n-Acetyl-cysteine, when administered orally or intravenously, is an antioxidant which enhances methylmercury excretion and helps restore the glutathione system when given either orally or intravenously [27-29].
Risk versus benefit of fish consumption from the Pacific basin
Seafood products are regularly sold in the US, and the average levels of mercury in different species of fish have been published by both the United States Environmental Protection Agency and the United States Food and Drug Administration [28]. As seen in Figure 3, there is a significant difference in the mercury levels measured from Pacific basin-caught and Atlantic basin-caught fish when compared to each other and to the USDA’s generalized published levels, in the same species of Tuna and Swordfish. This is important to note when taking into consideration various recommendations for seafood consumption. Given the apparently higher level of mercury possible in Pacific basin fish, recommendations for consumption of fish species from this area should likely be less than those caught in the Atlantic basin. Further research is needed to determine the potential toxicity of fish based on origin, size, migratory patterns and capture locations, among many other factors. Fortunately, the Minamata Convention has begun to tackle this complex problem by identifying and eliminating potential sources of mercury toxicity across the globe. Nonetheless, consumers need to exercise additional caution when consuming fish from these locations.
Had the subject patient wanted to supplement his protein sources with seafood, the best sources include (but are not limited to) anchovy, herring, salmon, catfish, clams, cod, crab, sardines, oysters, shrimp, tilapia, trout, whitefish, and Pollock. Fish that should be limited to one serving a week include (but are not limited to) bluefish, carp, grouper, halibut, mahi mahi, monkfish, tilefish (Atlantic ocean), tuna “albacore”, yellowfin tuna, striped bass (ocean) [29].
Lastly, fish to be avoided entirely due to their high levels of mercury include king mackerel, marlin, orange roughy, shark, swordfish, tilefish and bigeye tuna. However depending on further research, geographical origin of the species may need further consideration in these recommendations. There is a great need for local and national public health officials and the various stores Figure 3: Hg levels in Fish- Hg levels in fish shown were measured by ICP-MS. Pacific basin fish consumed by the patient (white bars) all showed dramatically elevated levels of total Hg, particularly swordfish. For comparison, USDA published levels of Hg in the same species of fish are shown in the black bars. The grey bars show measured levels of Hg in Atlantic basin-caught fish, which are within the USDA published ranges. and markets where consumers purchase fresh and frozen fish, to provide the public with information on mercury content, and allowable amount and frequency that mercury-laden fish should be consumed, allowing consumers to make informed decisions about which fish to eat.
As of August 24, 2020, the patient reports having no further complications with methylmercury toxicity. He reports that he no longer eats tuna or swordfish and receives his protein from eggs and occasional salmon steaks and shrimp.
Citation: Palmieri JR, Council-Troche M, Fraim J, McCabe A, Rzigalinski BA (2020) Chronic Methyl Mercury Toxicity in a 72 Year-Old Athletic Male from Southwestern Virginia. J Clin Toxicol. 10:454. DOI: 10.35248/2161-0495.20.10.454
Received: 31-Aug-2020 Accepted: 14-Sep-2020 Published: 21-Sep-2020 , DOI: 10.35248/2161-0495.20.10.454
Copyright: © 2020 Palmieri JR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.