Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2015) Volume 4, Issue 3
Resorcinol is widely used in manufacturing of several drugs and pharmaceutical products that are mainly used for topical ailments. The main objective of this study is to use an alternative strategy i.e., biofield treatment to alter the physical, spectral and thermal properties of resorcinol. The resorcinol sample was divided in two groups, which served as control and treated group. The treated group was given biofield treatment and both groups i.e., control and treated were analysed using X-ray diffraction (XRD), Fourier transform-infrared (FT-IR) spectroscopy, UV-Visible (UVVis) spectroscopy, Differential scanning calorimetry (DSC) and Thermogravimetric analysis (TGA). The results showed a significant decrease in crystallite size of treated sample i.e., 104.7 nm as compared to control (139.6 nm). The FTIR and UV-Vis spectra of treated sample did not show any change with respect to control. Besides, thermal analysis data showed 42% decrease in latent heat of fusion. The onset temperature of volatilization and temperature at which maximum volatilization happened was also decreased by 16% and 12.86%, respectively. The significant decrease in crystallite size may help to improve the spreadability and hence bioavailability of resorcinol in topical formulations. Also increase in volatilization temperature might increase the rate of reaction of resorcinol when used as intermediate. Hence, biofield treatment may alter the physical and thermal properties of resorcinol and make it more suitable for use in pharmaceutical industry.
Keywords: Resorcinol; Biofield energy treatment; X-Ray diffraction; Fourier transform infrared spectroscopy; Ultraviolet-Visible spectroscopy; Differential scanning calorimetry; Thermogravimetric analysis
XRD: X-Ray Diffraction; FT-IR: Fourier Transform Infrared; DSC: Differential Scanning Calorimetry; TGA: Thermogravimetric Analysis; DTG: Derivative Thermogravimetry; NCCAM: National Centre for Complementary and Alternative Medicine
Resorcinol is a dihydric phenol having the hydroxyl group at 1 and 3 positions in the benzene ring [1]. It occurs naturally in argan oil as main natural phenol. It is white crystalline powder having a faint odour and bitter-sweet taste [2]. It is used as a chemical intermediate in manufacturing of pharmaceuticals, dyestuffs and fungicides such as p-aminosalicylic acid, hexylresorcinol and light screening agents for protecting plastics from UV lights [3,4]. It is used in the formulation of several pharmaceuticals such as acne creams, hair dyes, anti-dandruff shampoos, and sun tan lotions. It also possesses various therapeutic uses such as topical antipruritic and antiseptic. It is used to treat seborrheic dermatitis, psoriasis, corns, warts and eczema. It is effective in the treatment of several dermatological problems due to its antibacterial, antifungal and keratolytic effects [5,6]. Resorcinol solution in ethyl alcohol (Jessner’s solution) is used in chemical peeling [7], and it has special medical use as biological glue (gelatin-resorcinol-formaldehyde glue) in cardiovascular surgery [8,9]. Despite its wide pharmaceutical applications, some side effects are also associated with it, for instance, mild skin irritation, skin redness, etc. It is also hygroscopic i.e., absorb moisture from the air and turns pink on exposure to air or light [10]. By conceiving the usefulness of resorcinol, the present study was attempted to investigate an alternative way that can improve the physical and thermal properties of resorcinol. In recent years, biofield treatment was proved to be an alternative method that has an impact on various properties of living organisms and non-living materials in a cost effective manner. It is already demonstrated that energy can neither be created nor be destroyed, but it can be transferred through various processes such as thermal, chemical, kinetic, nuclear, etc. [11-13]. Similarly, electrical current exists inside the human body in the form of vibratory energy particles like ions, protons, and electrons and they generate a magnetic field in the human body [14,15]. This electromagnetic field of the human body is known as biofield, and energy associated with this field is known as biofield energy [16,17]. The human beings are infused with this precise form of energy, and it provides regulatory and communications functions within the organism [18,19]. The health of living organisms can be affected by balancing this energy from the environment through natural exchange process [20]. National Centre for Complementary and Alternative Medicine (NCCAM), which is part of the National Institute of Health (NIH) places biofield therapy (putative energy fields) as subcategory of energy medicine among complementary and alternative medicines. The healing therapy is also considered under this category [21,22]. Thus, the human has the ability to harness the energy from environment or universe and can transmit it to any living or non-living object. This process is termed as biofield treatment. Mr. Trivedi’s unique biofield treatment (The Trivedi Effect®) is well known and significantly studied in different fields such as microbiology [23-25], agriculture [26-28], and biotechnology [29,30]. Recently, it was reported that biofield treatment has changed the atomic, crystalline and powder characteristics as well as spectroscopic characters of different materials. Moreover, alteration in physical, thermal and chemical properties were also reported in materials like antimony, bismuth and ceramic oxide [31,32]. Hence, based on above results the current study was designed to determine the impact of biofield treatment on physical, spectral and thermal properties of resorcinol.
Study design
Resorcinol was procured from Loba Chemie Pvt. Ltd., India. The sample was divided into two parts and referred as control and treatment. The treatment sample in sealed pack was handed over to Mr. Trivedi for biofield treatment under standard laboratory conditions. Mr. Trivedi provided the treatment through his energy transmission process to the treatment group without touching the sample. The biofield treated sample was returned in the same sealed condition for further characterization using XRD, FT-IR, UV-Vis, DSC, and TGA techniques. For determination of FT-IR and UV-Vis spectroscopic characters, the treated sample was divided into two groups i.e., T1 and T2. Both treated groups were analysed for their spectral characteristics using FT-IR and UV-Vis spectroscopy as compared to control resorcinol sample.
X-ray diffraction (XRD) study
XRD analysis was carried out on Phillips, Holland PW 1710 X-ray diffractometer system. The X-ray generator was equipped with a copper anode with nickel filter operating at 35 kV and 20 mA. The wavelength of radiation used by the XRD system was 1.54056 Å. The XRD spectra were acquired over the 2θ range of 10°-99.99° at 0.02° interval with a measurement time of 0.5 second per 2θ intervals. The data obtained were in the form of a chart of 2θ vs. intensity and a detailed table containing peak intensity counts, d value (Å), peak width (θ°), and relative intensity (%).
The average size of crystallite (G) was calculated from the Scherrer equation [33] with the method based on the width of the diffraction patterns obtained in the X-ray reflected crystalline region.
G=kλ/(bCosθ)
Where, k is the equipment constant (0.94), λ is the X-ray wavelength (0.154 nm), B in radians is the full-width at half of the peaks and θ the corresponding Bragg angle.
Percent change in crystallite size was calculated using the following equation:
Percent change in crystallite size=[(Gt-Gc)/Gc] ×100
Where, Gc and Gt are crystallite size of control and treated powder samples, respectively [34].
Fourier transform-infrared (FT-IR) spectroscopic characterization
The powdered sample was mixed in spectroscopic grade KBr in an agate mortar and pressed into pellets with a hydraulic press. FTIR spectra were recorded on Shimadzu’s Fourier transform infrared spectrometer (Japan). FT-IR spectra are generated by the absorption of electromagnetic radiation in the frequency range 4000-400 cm-1. The FT-IR spectroscopic analysis of resorcinol (control, T1 and T2) was carried out to evaluate the impact of biofield treatment at atomic and molecular level like bond strength, stability, rigidity of structure etc. [35].
UV-Visible spectroscopic analysis
The UV-Vis spectral analysis was measured using Shimadzu UV-2400 PC series spectrophotometer. It involves the absorption of electromagnetic radiation from 200-400 nm range and subsequent excitation of electrons to higher energy states. It is equipped with 1 cm quartz cell and a slit width of 2.0 nm. The UV-Vis spectra of resorcinol were recorded in methanol solution at ambient temperature. This analysis was performed to evaluate the effect of biofield treatment on the structural property of resorcinol sample. The UV-Vis spectroscopy gives the preliminary information related to the skeleton of chemical structure and possible arrangement of functional groups. With UVVis spectroscopy, it is possible to investigate electron transfers between orbitals or bands of atoms, ions and molecules existing in the gaseous, liquid and solid phase [35].
Differential scanning calorimetry (DSC) study
Differential scanning calorimeter (DSC) of Perkin Elmer/Pyris-1 was used to study the melting temperature and latent heat of fusion (ΔH). The DSC curves were recorded under air atmosphere (5 mL/ min) and a heating rate of 10°C/min in the temperature range of 50°C to 350°C. An empty pan sealed with cover pan was used as a reference sample. Melting temperature and latent heat of fusion were obtained from the DSC curve.
Percent change in latent heat of fusion was calculated [36] using following equations to observe the difference in thermal properties of treated resorcinol sample as compared to control:
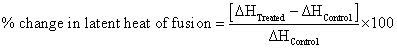
Where, ΔH Control and ΔH Treated are the latent heat of fusion of control and treated samples, respectively.
Thermogravimetric analysis/Derivative thermogravimetry (TGA/DTG)
Thermal stability of control and treated samples of resorcinol was analysed by using Mettler Toledo simultaneous Thermogravimetric analyser (TGA/DTG). The samples were heated from room temperature to 400°C with a heating rate of 5°C/min under air atmosphere. From TGA curve, onset temperature Tonset (temperature at which sample start losing weight) and from DTG curve, Tmax (temperature at which sample lost its maximum weight) were observed [37].
Percent change in Tmax was calculated using following equation:
% change in Tmax = [(Tmax, treated-Tmax, control)/ Tmax, control] ×100
Where, Tmax, treated and Tmax, treated are the temperature at which sample lost its maximum weight due to volatilization in control and treated sample, respectively. Similarly, the percent change in onset temperature at which sample start losing weight was also calculated.
X-ray diffraction
X-ray diffraction analysis was conducted to study the crystalline nature of the control and treated samples of resorcinol. XRD diffractogram of control and treated samples of resorcinol are shown in Figure 1 and results are given in Table 1. The XRD diffractogram of control resorcinol showed intense crystalline peaks at 2θ equal to 18.04°, 18.18°, 19.11°, 19.68°, 19.93°, and 20.08°. The intense peaks indicated the crystalline nature of resorcinol. The XRD diffractogram of treated resorcinol showed the crystalline peaks at 2θ equal to 18.04°, 18.18°, 19.19°, 20.04° and 29.73°. The peaks in treated sample showed high intensity as compared to control that indicated that crystallinity of treated resorcinol sample increased along the corresponding plane as compared to the control. It is presumed that biofield energy may be absorbed by the treated resorcinol molecules that may lead to form a symmetrical crystalline long range pattern that further results in increasing the symmetry of resorcinol molecules. Besides, the crystallite size was found to be 139.6 nm in control sample whereas, it was reduced to 104.7 nm in treated resorcinol. The crystallite size was reduced by 25% in treated resorcinol as compared to control. Other parameters like the volume of unit cell and molecular weight showed very slight change (0.05%) as compared to control sample. The effect of biofield treatment on crystallite size was also reported previously [37,38]. It is hypothesized that biofield treatment might produce the energy that causes the fracturing of grains into subgrains hence; the crystallite size was decreased in treated sample as compared to control. As resorcinol is used in many topical formulations, the decrease in crystallite size may improve its spreadability over the skin that further affects its bioavailability [39]. Hence, the treated resorcinol with decreased crystallite size may improve its bioavailability when used in topical formulations.
| Parameter | Control | Treated |
|---|---|---|
| Volume of unit cell×10-23 (cm3) | 57.162 | 57.190 |
| Crystallite size (nm) | 139.60 | 104.70 |
Table 1: XRD analysis of control and treated samples of resorcinol.
FT-IR analysis
The FT-IR spectra of control and treated (T1 and T2) samples are shown in Figure 2. The spectra showed characteristic vibrational frequencies as follows:
Carbon-Hydrogen vibrations: The aromatic structure of resorcinol showed the presence of C-H stretching vibrations in the region 3100-3000 cm-1 which was the characteristic region. The frequency of C-H stretching was overlapped with O-H stretching frequencies in all three samples, i.e., control, T1 and T2. The C-H in-plane bending vibrations were observed at 1379 cm-1 in control and T1 sample whereas, at 1381 cm-1 in T2 sample. The C-H outof- plane bending vibrations appeared at 773 cm-1 in control and T1 sample whereas, at 777 cm-1 in T2 sample.
Oxygen-Hydrogen vibrations: In the present study, the O-H stretching vibration was observed at 3257-3207 cm-1 in control sample whereas at 3263-3200 cm-1 in T1 and 3281-3072 cm-1 in T2 sample. Generally the O-H band were appeared at frequency range 3600-3300 cm-1; however, broadening of the peak may occur in the presence of H-bonded O-H stretching. Hydrogen bonding may shift the peaks to lower frequencies as it was seen in FT-IR spectra of control and treated samples of resorcinol. Hence, it confirmed the presence of H-bonding on resorcinol sample.
C-OH group vibration
The most important peaks due to C-OH stretching mode were appeared at 1311-1298 cm-1 and 1166-1151 cm-1 as doublet peak in the control sample. In T1 sample, the peaks were appeared at 1310- 1298 cm-1 and 1166-1151 cm-1 whereas; in T2 sample the peaks were appeared at 1300-1284 cm-1 and 1166-1143 cm-1. The C-OH bending peak was appeared at 462 cm-1 in all three samples i.e., control, T1, and T2.
Ring vibration
The fundamental vibrational modes of C-C stretching generally occurred in the region of 1600-1400 cm-1. In the present study, the peaks observed at 1608 and 1489 cm-1 in control and T1 sample were assigned to C-C stretching vibrations. Whereas, in T2, these peaks were appeared at 1604 and 1487 cm-1. Another peak due to ring vibration was appeared at 545 cm-1 in all three samples, i.e., control, T1, and T2. The other important peaks were appeared at 842 and 740 cm-1 due to meta di-substituted ring in control and T1 sample. Whereas, the same peaks were appeared at 844 and 742 cm-1 in T2 sample. The overall analysis was supported by literature data [4] and showed that there was no significant difference between observed frequencies of control and treated (T1 and T2) samples. Hence, it showed that biofield treatment might not induce any significant change at bonding level.
UV-Vis spectroscopic analysis
The UV spectra of control and treated samples (T1 and T2) of resorcinol are shown in Figure 3. The UV spectrum of control sample showed absorption peaks at λmax equal to 205, 275 and 281 nm and was well supported by literature [40]. The absorbance peaks were appeared at the same wavelength in treated samples. In T1 sample, the peaks were found at λmax equal to 204, 275 and 281 nm and in T2 sample, they were appeared at λmax equal to 205, 275 and 281 nm. It showed that no change was found in UV spectroscopic properties, i.e., related to structure skeleton, functional groups or energy for electron transfers between orbitals or bands of atoms of treated resorcinol as compared to control.
Thermal studies
DSC analysis: DSC was used to determine the latent heat of fusion (ΔH) and melting temperature in control and treated samples of resorcinol. The DSC analysis results of control and treated samples of resorcinol are presented in Table 2. In a solid, the amount of energy required to change the phase from solid to liquid is known as the latent heat of fusion. The result showed that ΔH was decreased from 179.77 J/g (control) to 103.47 J/g in treated resorcinol. It indicated that ΔH was decreased by 42.45% in treated sample as compared to control. It was previously reported that resorcinol molecules possess rigid structure but as the temperature increases, this rigidity breaks down. The molecules rearrange into a hydrocarbon resembling structure and achieve lower van der walls interactions [41]. Hence, it is hypothesized that biofield treatment might produce the energy. This energy probably causes deformation of hydroxyl bond in treated resorcinol, and it needs less energy in the form of ΔH to undergo the process of melting. Previously, our group reported that biofield treatment has altered ΔH in lead and tin powder [42]. Moreover, the melting temperature of treated (112.56°C) sample showed very slight change with respect to control (111.18°C) resorcinol sample.
| Parameter | Control | Treated |
|---|---|---|
| Latent heat of fusion ΔH (J/g) | 179.77 | 103.47 |
| Melting point (°C) | 111.18 | 112.56 |
| Onset temperature (°C) | 200 | 168 |
| Tmax(°C) | 217.11 | 189.2 |
Table 2: Thermal analysis of control and treated samples of resorcinol. Tmax: Temperature at which maximum weight loss occur.
TGA/DTG analysis: TGA/DTG of control and biofield treated samples are summarized in Table 2. TGA thermogram (Figure 4) showed that control resorcinol sample started losing weight around 200°C (onset) and stopped around 246°C (end set) which could be due to volatilization of resorcinol [43]. However, the treated resorcinol started losing weight around 168°C (onset) and terminated around 215°C (end set). It indicated that onset temperature of treated resorcinol was decreased by 16% as compared to control. Besides, DTG thermogram data showed that Tmax in control sample was 217.11°C and in treated sample, it was found at 189.2°C. It showed that Tmax was decreased by 12.86% in treated sample as compared to control. Furthermore, the reduction in onset temperature and Tmax in treated resorcinol with respect to control sample may be correlated with the increase in volatilization of treated resorcinol after biofield treatment. A possible reason for this reduction is that biofield energy might cause some alteration in internal energy which probably resulted into earlier volatilization of treated resorcinol sample as compared to control. Also, decrease in volatilization temperature indicated that resorcinol molecules change their phase from liquid to gas at low temperature, which may results in fasten the rate of those reactions where resorcinol can be used as an intermediate in synthesis [44]. Hence, overall observations suggest that biofield treated resorcinol can be used to enhance the reaction kinetics and yield of the end product.
The XRD results showed the decrease in crystallite size (25%) in treated sample as compared to the control that may occur due to biofield treatment that probably produces the energy which leads to fracturing of grains into subgrains. The reduced crystallite size of treated resorcinol sample may be used to improve its bioavailability in topical preparations. The DSC analysis of treated sample showed 42.45% decrease in ΔH value as compared to control, which probably occurred due to deformation of hydroxyl bond in treated sample. The biofield treatment might affect the structure rigidity of resorcinol and hence reduced the latent heat of fusion. TGA/DTG analysis revealed that onset temperature of volatilization and Tmax were decreased by 16% and 12.86%, respectively. This reduction in volatilization temperature of treated sample might be helpful for resorcinol to be used as a chemical intermediate in the synthesis of various pharmaceuticals. Hence, above study concluded that biofield treatment might alter the physical and thermal properties of resorcinol that could make it more useful in pharmaceutical industries by increasing the bioavailability and reaction kinetics.
The authors would like to acknowledge the whole team of Sophisticated Analytical Instrument Facility (SAIF), Nagpur and MGV Pharmacy College, Nashik for providing the instrumental facility. We are very grateful to Trivedi Science, Trivedi Master Wellness and Trivedi Testimonials for their support in this research work.