
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2024)Volume 14, Issue 3
Numerous industrial operations discharge contaminants created by humans into rivers. When these toxins are present in water, they pose a major danger to human health and the health of other ecosystems. Significant progress has been achieved in reducing the quantity of hazardous substances entering our rivers. Through the application of catalytic hydrogenation procedures, it is feasible to remediate and lessen the hazards of both Nitrophenols (NP) and Methylene Blue (MB) in water. The hydrogenation reactions between the dye and the NP were investigated utilizing a variety of noble metal (Au, Pd) and noble metal (Pt) nanoparticle-based catalysts. Recently, activated carbon-based catalysts have garnered considerable attention. Activated Carbon (AC) is a substance that is mainly amorphous, very porous, and highly adsorbent. The majority of the molecule is composed of aromatic carbon atoms that are crosslinked. Modifications to the synthesis and post-treatment conditions may modify the textural qualities. However, in terms of gold and platinum dispersion and size restrictions, Activated charcoal has been categorized only as a palladium supporting material. This work used a homogenous deposition approach to fabricate Au/AC, a mesoporous activated carbon substrate. The synthesized material was analyzed using FTIR and SEM analysis. The catalyst generated from the hydrogenation of P-Nitrophenol (PNP) and MB was tested for its catalytic potential for 5 to 13 minutes respectively. PNP and MB were completely hydrogenated. During the use of the synthesized materials, the decontamination of two dangerous organic pollutants, PNP and MB dye, were examined. The purpose of this study was to determine the reusability of materials in real waste water treatment applications that required more than one cycle.
Gold based activated charcoal; Organic pollutant; Catalytic degradation; Dye
Industrial wastes stand as the major source of water pollution, emanating a plethora of organic pollutants with deleterious implications for both the environment and human health. Understanding the health effects of these synthetic toxins at the minute concentrations encountered in industrial wastewater remains nascent. Nonetheless, it is established that water sources are repositories of considerable industrial effluent and toxic contaminants, prompting concerns regarding their discharge into aquatic ecosystems [1]. The imperative to eliminate anthropogenic contaminants from water prior to domestic utilization necessitates a meticulous evaluation of the costeffectiveness of water treatment methodologies. Thus, there is a compelling demand to augment technical acumen and devise protocols that not only demonstrate environmental compatibility but also anticipate their repercussions on marine pollution. This perseverance is underscored by the recognition of substantial industrial effluent and pollutant presence in water sources, warranting scrupulous consideration prior to their release into aquatic streams [2].
Several techniques are employed for the removal of color from industrial effluents, encompassing biological treatment, coagulation, flotation, adsorption, oxidation, and hyper filtration. Among these methodologies, adsorption has emerged as a particularly effective and economically viable approach for decolorizing textile wastewater [3]. Diverse adsorbents have found application in removing various substances, including dyes, metal ions, and other organic materials, from aqueous solutions. Examples of such adsorbents comprise perlite, bentonite, silica gels, fly ash, lignite, peat, and silica. Activated carbon, distinguished by its structurally homogeneous composition, extensive surface area, microporous structure, and radiation stability, stands out as a pivotal material in industrial processes [4]. It is widely employed as an adsorbent, catalyst, or catalyst support. The adsorption characteristics of activated carbon are contingent upon factors such as particle size, porosity, ash content, degree of carbonization, and the method of activation.
Activated charcoal was previously thought to be the ultimate antidote [5]. It is still promoted as a powerful natural treatment today. It is said to provide a range of health advantages, including reducing cholesterol, whitening teeth, and healing hangovers activated charcoal isn't similar to charcoal briquettes, which might be used to hearth place your grill [6]. Charcoal briquettes have now no longer been activated at excessive temperatures, notwithstanding the truth that they will be made from the equal base ingredients. Furthermore, they consist of different human-poisonous chemicals [7].
By using fossil fuels, people are posing an ever-growing threat to the ecosystem, wreaking havoc on our world in the long run [8]. noxious; industrial discharges of toxic effluents into bodies of water, degrading their value and endangering aquatic life; urbanization leading to deforestation and hence poor air quality; soil productivity loss owing to plastic trash, to name a few examples [9]. Despite all of these simultaneous effects, little or no effort has been made to address pollution's environmental consequences and threats to human health in a planned and pragmatic way [10]. Waste drainage from different industries is the main cause for water pollution. Pollution may be a significant hazard to human life, particularly when water is utilized for drinking and other household functions [11]. Contaminated water has been associated with the spread of illnesses such as cholera, typhoid fever, and TB [12]. Massive oil spills produced by ships or broken oil pipelines in the oil industry are detrimental to ocean plants, mollusks, and other marine species that supply food for peoples [13]. When pesticides such as DDT are released into the environment, they may accumulate in the food chain, posing a significant threat to aquatic life [14].
Amongst inorganic sources arsenic, chromium, lead, nickel and mercury have been shown to be more toxic to humans [13]. When you drink water, you are ingesting a lot of arsenic contaminants. The production of red and white blood cells may be impaired, blood vessels may be constricted, and tingling may occur in the fingers and toes [14]. The heartbeat may be disturbed, and the digestive system may be affected by arsenic poisoning [15]. Chromium may be found in water and soil causing cancer, infections, skin diseases, respiratory disorders as well as concerns with the excretory, reproductive, and digestive systems [16]. Nickel (Ni), also known as the "Allergen of the Year 2008," is a metal that may be found in water, air, and soil. It can lead to skin infections, heart problems, renal problems, and lung cancer, among other things [17]. Lead (Pb) is a toxic metal that can be found in trace amounts in soil and water. Vehicle exhaust, domestic paint, and industrial waste products are only a few of the sources of Pd in drinking water. Lead's physiological roles in the human body are unknown [18]. Mercury is a naturally occurring element that is a hazardous metal that may affect both animals and people's health [19]. Dyes based industries add their contaminants into water bodies. These Dyes have chromophores and auxochromes which can be answerable for their color and substantively [20]. Tints include certain chemical components that are hazardous to living beings. Humans are also exposed to certain dyes, which can cause cancer, allergies, skin irritation, mutations, and dermatitis [21].
Several industrial discharge trashes directly into fresh water, causing diarrhea, skin rashes, vomiting, respiratory issues, eye irritation, and perhaps tumors [22]. Several techniques have been discussed in the literature for the reduction of anthropogenic pollutants using expensive catalysts however. Activated charcoal was previously thought to be the ultimate antidote [23]. It is still promoted as a powerful natural treatment today. It is said to provide a range of health advantages, including reducing cholesterol, whitening teeth, and healing hangovers activated charcoal isn't similar to charcoal briquettes, which might be used to hearth place your grill [24]. Charcoal briquettes have now no longer been activated at excessive temperatures, notwithstanding the truth that they will be made from the equal base ingredients. Furthermore, they consist of different human-poisonous chemicals [22]. In the current study catalytic reduction of P-Nitrophenol to P-Aminophenol, MB, and Leuco Methylene Blue (LMB) has been discussed over a cheap gold based AC.
Chemicals and reagents
All of the chemicals used in this study were of analytical grade and weren't further purified prior usage. Hydrogen Tetrachloroaurate (III) Hydrate (HAuCl4.3H2O), NaBH4, was purchased from Merck. Sodium hydroxide (NaOH) from sigma Aldrich; and activated carbon, and de-ionized H2O.
Synthesis of Au/activated carbon
The composite Au/Ac was synthesized using the impregnation process. In a typical experiment the (HAuCl4) salt was dissolved in 10 mm of double distilled water and mixed with activated carbon powder. It was then subjected to stirrer for about 24 hours at and after the final composite was dried in oven at 100°C for 20 hours. Various concentration of the Au/Ac like 1%, 0.5%, and 0.25% were employed. The color change from red to brown is an indication of the Au in the composite [25].
Catalyst preparation
Catalyst was produced by deposition. Adsorption of metal salt (HAuCl4) into the support solution occurred as a result of interactions between metallic compound ions and active sites on the supporting material. Excess solvent may be removed by using thermal deposition. Ionic species (Au3+) in the solution cause ion exchange between supporting material and active component. By stabilizing the metallic component by adsorption contact, this technique of catalyst synthesis not only produces low catalyst charges but also avoids the production of subsequent catalysts [26]. A thin layer of catalyst was deposited on the surface of the supporting material as a consequence. One of the best uses for this technique is the deposition of precious metals surface dispersion may be obtained even with a little quantity of catalyst (Figure 1).
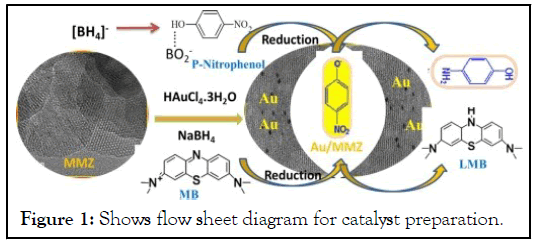
Figure 1: Shows flow sheet diagram for catalyst preparation.
Catalytic experiment
The catalytic activity of the catalyst was examined through the reduction of P-NP to P-AP (0.005 M) using NaBH4 (0.2 M) as the reducing agent. On the next step, 2.5 milliliters of 2 mg aqueous Au catalyst scattering were added. We used UV-visible spectroscopy to measure the solution after it had been mixed. According to the UV-visible spectrum, P-NP had a peak at 400 nanometers, whereas P-AP had one at 296 nm. Second, the hydrogenation of MB to LMB was detected using a UV-visible spectrophotometer. The earlier procedure called for injecting a 25 ppm methylene blue solution into which 1 gram of catalyst was dissolved. A UV-visible spectrophotometer with 0.2 milliliters of sodium borohydride was used to analyze the combination (0.2 M). MB used to have a peak in the UV-visible spectrum at 664 nm, however that peak has now vanished and been replaced by another.
Hydrogenation experiment
The first step will be to examine gold's capacity to facilitate the conversion of P-Nitrophenol to P-Aminophenol. The reaction will be monitored using a UV-visible spectrophotometer. PNitrophenol solution in water and 0.25 ml fresh NaBH4 solution will be combined (0.2 M). After that, added 2.5 mL of gold catalyst aqueous dispersion (2 mg). To ensure that the solution has been thoroughly dissolved, UV-visible measurements will be carried out. P-Nitrophenol peaks at 400 nm in the UV-visible spectrum, while P-Aminophenol peaks at 296 nm. Leucomethylene blue may be made by using an ultraviolet-visible spectrophotometer to convert methylene blue. NaBH4 (0.2 milliliters) and methylene blue solution (25 parts per million) are used to dissolve two milligrams of catalyst in 2.5 milliliters of the solution (0.2 M). An ultraviolet spectrophotometer is then used to measure the solution's concentration in UV light. When the peak of the UV-visible spectrum at 664 nanometers fades, it is replaced by leuco methylene blue. Researchers employed activated carbonsupported metals to study gold's relative activity for hydrogenation of ethylene using Schuit and van Reijen. Bond has written on Beeck's work in this journal. In both tests, platinum was shown to be more active than palladium, but gold was found to be more active than palladium. The proportion dcharacter of the metallic link has an impact on the activity of these metals as well [27]. This reaction has lower activation energy, but the potential energy of both the reactant and the product is not changed. Platinum, palladium, and gold are among the most often used catalysts for hydrogenation. According to the diagram, catalytic hydrogenation takes place in at least two stages. Alkenes and hydrogen must be absorbed on the catalyst's surface before they can be employed.
Characterization
The dispersion degree of gold nanoparticles was analyzed by Transmittance electronic microscope. The surface area of the catalyst was analyzed by Brunauer Emmett and Teller (BET). The crystallinity of the catalyst was analyzed by X-Rays Diffraction (XRD). Furthermore the catalyst was characterized by XPS, UV-DRS, FTIR, SEM.
Selected metal concentrations were determined by ICP-AES (Sii Nano Technology Inc.) instrumentation. The inner surface of catalytic tubular reactors was examined using a scanning electron microscope and an energy-dispersive X-ray spectrometer (EDX, XL30S; Philips Co.). At room temperature, UV-vis absorption spectra were recorded using a spectrophotometer (Hitachi U-3310). Belsorp MAX was used to quantify the volumetric specific surface area and pore diameter of porous Pd (N2 adsorption). Preliminary degassing of the samples was place in an ultra-high vacuum at temperatures of 250°C.
Catalytic hydrogenation of P-Nitrophenol and methylene activity
The catalytic activity of the synthesized composite was checked via the catalytic reduction of P-NP to P-AP [28]. In a typical experiment P-NP solution (0.005 M) was mixed with the known volume of NaBH4 (0.2 M). The aqueous dispersion of Au catalysts (2 mg) was then added to the solution (2.25 mL). In this case, the reaction time t=0 minutes calculated from UV-vis measurements of the combined solution is based on this data. After that, Uv-vis measurements were employed to collect reaction data. NaBH4 solution (0.2 M) was used to mix with 10.0 mL of an aqueous P-NP solution to see whether the solution could be reused or not (0.005 M). Afterwards, 125.0 mL of the aqueous dispersion of the Au catalysts (100 mg) was added to the mixture. A centrifuge was used to extract the catalyst from the reaction mixture once the hydrogenation process was complete. The recovered catalyst was rinsed three times with deionized water. The catalyst from an earlier cycle was used in this one. After 15 minutes of reaction, the solution was observed by UV-vis spectroscopy. Testing of Au catalysts was also done by hydrogenating MB into LMB. In a single process, NaBH4 solution was injected immediately after 1 mg of Au catalysts were distributed in 2.5 mg of MB dye solutions (25 ppm) (0.2 M). The reaction mechanism was previously claimed to be visible. NaBH4 solution was used for the recycling experiment, which included 25 ppm MB and 50 mg of Au catalysts in 125.0 mL of NaBH4 (0.2 M). The catalyst was removed from the reaction mixture when hydrogenation was complete. The recovered catalyst was cleaned in deionized water for the next batch. UV-vis spectroscopy was used to monitor the fluid after a 4-minute reaction. The operation was repeated six times. In this study, 0.25 percent of the entire population was used as a sample size for this approach.
FTIR Analysis
The fixed mix of functional groups might be used to analyze the spectra of activated carbon samples. The phenolic ester has OH and NH stretching between 3100 and 3500 cm-1, C-H aromatic between 3000 and 3100 cm-1, C-H aliphatic between 2800 and 3000 cm-1, C=O and C-O stretching between 1640 and 1750 cm-1 [29] and linked ketonic structures and carboxylic acid stretching between 1640 and 1750 cm-1. Between 700 and 900 cm-1, various bands associated to aromatic, out of plane C-H bending with varied degrees of substitution can be detected [30]. After connecting to gold nanoparticles, these bands correspond to C-H stretch vibrations from aryl and methylene C-H stretching vibrations, and they are shifted to 3062, 3030, 2926, and 2867 cm-1, have discovered that activated carbons hydroxyl groups can be used to decrease Au3+ ions, with the hydroxyl groups being oxidized to carbonyl groups [31]. As a result, a new band of about 1732 cm-1 that correlates to carbonyl groups has been discovered. The band at 1126 cm-1 in curve 1 corresponds to the C-O stretching vibration frequency, with absorption bands at 1114 cm-1 for gold nanoparticles generated in the sample (Figure 2) [32].
Figure 2: FTIR analysis of 0.5% Gold/Activated carbon, 0.25%Gold/Activated carbon, 1% Gold/Activated carbon.
The SEM analysis of activated and Au based composite is shown in Figure 3. It is found that AC exhibits morphological behavior of particles with vascular characteristics. The Au were impregnated on activated carbon lots of change occur in the morphology, that the Au NPS are small and spherical in shape, and are well dispersed without any agglomeration. From the SEM micrographs, the crystal morphology of 0.5 Au/AC, 0.25 Au/AC and 1% Au/AC is observed which indicates the dispersion of Au over activated carbon as reported [33]. The deposition of Au NPs within the interspace between the layers of support materials could be reflected in the shape of the activated carbon surface.
Figure 3: (a) Activated carbon (b) 0.25% Au/AC (c) 0.5% Au/AC (d) 1.0% Au/A.
Catalytic hydrogenation of PNP to P-AP
The catalytic activity of the synthesized Au-based composite was checked through the catalytic conversion of P-NP to P-AP using NaBH4 as reductant (Figure 4). At time t=0 and at initial concentration (Co) there observed no change in the λmax and by introducing the composite after sometime that when t=19 minutes at final concentration (Ct) there occurred decrease in the absorbance exhibited the P-NP to P-AP conversion which was verified via their λmax observed at 400 nm. Moreover it was also confirmed that the composite without Au particles showed no catalytic activity and when present indicated more activity by easily and quickly conversion of P-NP to P-AP. So it was concluded finally that our composite is the best catalyst as compared to their individual components (Figure 5).
Figure 4: Shows reaction method for preparation of catalyst.
Figure 5: (a) Show reduction of P-NP and (b) Show rate of
reaction. Note: 
Catalytic hydrogenation of MB
For the first time, the catalytic hydrogenation of MB to LMB in the presence of NaBH4 was employed to assess Au-based catalyst activity (Figure 6). The UV-vis spectrum of the dark blue initial MB solution reveals an adsorption peak with a maximum at 664 nm. With time=0, there is no change in peak intensity after adding catalysts to the MB and NaBH4 solutions, but as time passes, the peak at 664 nm fades, suggesting that MB hydrogenation has happened. After adding Au to activated carbon in varied loading levels, hydrogenation of MB was discovered. The 0.25 percent Au/AC samples showed the best recyclability and reusability among the 0.5 percent and 1 percent Au/AC samples. The 0.25 Au/AC sample may completely hydrogenate MB and be reused multiple times.
Figure 6: Shows catalytic hydrogenation of MB.
To produce metal-based AC materials, such Au, a mutual process of dissolving and self-assembly was used, using advanced technologies like as XRD, SEM, TEM, XPS, and FTIR. It is reasonable to claim that several approaches have produced large surface area and well-structured micro-mesoporous composite materials. For both methylene blue and p-nitrophenol,, it was found that Au/AC had the maximum reduction ability for both methylene blue and p-nitrophenol, removal from water. The catalyst generated from the hydrogenation of PNP and MB was tested for its catalytic potential. In 13 minutes and 5 minutes, PNP and MB were completely hydrogenated, according to the results of the research. Although 0.25 percent Au/AC is less active than 0.5% and 1.0% Au/AC, its wide surface area and big pores make it more effective. Stability and regeneration may be shown by the catalyst's capacity to preserve its porous structure throughout the reaction process. It is possible that the hydrogenation of PNP and MB occurs in a pseudo first order manner, based on the kinetic study of the reaction.
All authors declare that there are no financial relationships or affiliations that could be perceived as a competing interest in connection with the research presented in this article. Furthermore, we declare that there were no personal or professional relationships that could influence the study's findings.
The authors declare no conflicts of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Shah NA, Ullah S, Hussain S, Akitsu T (2024) Catalytic Reduction of Anthropogenic Pollutants over Gold (Au) Based Activated Charcoal. J Clin Toxicol. 14:554.
Received: 26-Dec-2023, Manuscript No. JCT-23-28624; Editor assigned: 28-Dec-2023, Pre QC No. JCT-23-28624 (PQ); Reviewed: 11-Jan-2024, QC No. JCT-23-28624; Revised: 18-Jan-2024, Manuscript No. JCT-23-28624 (R); Published: 25-Jan-2024 , DOI: 10.35248/2161-0495.24.14.554
Copyright: © 2024 Shah NA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.