Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2012) Volume 1, Issue 1
Some volatile binary metal-organic frameworks of titanium (IV), [Ti(OOCR) 4 ] (where R = C 13 H 27, C 15 H 31, C 17 H 35 or C 21 H 43 ) were synthesized by the reaction of titanium tetrachloride and sodium salts of the straight chain fatty acids (prepared in situ) in 1:4 molar ratio. The isolated solid products were showing poor crystallinity and were characterized by their elemental analyses, molecular weight determinations, conductance, spectral (infrared, 1 H NMR, 13 C NMR, FAB mass and powder XRD) and TEM studies. Their monomeric nature was confirmed by molecular weight determinations and FAB mass spectra. Eight coordination number of titanium (IV) have been assigned in the isolated compounds. TEM indicated the particles are spherical in shape having ~200 nm diameter.
Keywords: Carboxylates; Metal-organic frameworks; Powder XRD; Titanium(IV)
Metal-organic frameworks (MOFs) of some transition metals have attracted a considerable attention in recent years [1-5] due to their wide range of applications. At present, various titanium-based materials have successfully been obtained in the form of nanotubes, nanofibers, nanowires, nanoflowers and nanocubes [6-8]. A key challenge for the industrial use of MOFs is to deliver them in a definite shape and size for their applications in many fields. There is an increasing trend in the research and development of TiO2 coatings through metallo-organic chemical vapour deposition (MOCVD) for their applications as being semi conductor and chemically stable under different conditions. The evolution of metal–organic complex precursors of Ti, owing to the limitations of the alkoxide or other precursors including halides, is well documented in the literature [9,10]. Therefore, a good air and moisture stable, easy-to-handle and volatile titanium precursor is a prerequisite for MOCVD process [11]. One of the most active field in titanium(IV) chemistry is in the design of new compounds using different substituents having anticancer activity [12-14]. It has also been observed that only oxo- or di- or tri- substituted carboxylate derivatives of titanium(IV) are resulted after a number of trials to synthesize its tetracarboxylates [15-17].
Keeping in view of these objectives, some titanium(IV) complexes of electron- rich ligands have been recently synthesized in our laboratory, which are reported to be hydrolytically stable [18,19]. In this paper we report an easier method to synthesize titanium(IV) tetracarboxylates and solid products have been isolated. These compounds are volatile in nature as well as hydrolytically stable, having Ti-O-C linkage, a basic requirement for catalytic action. These have been characterized by different spectral studies to arrieveat their structure and coordination behaviour of the ligands.
Materials and analytical methods
All the reactions were carried out under strictly anhydrous conditions. Glass apparatus with interchangeable quick fit joints were used throughout. Organic solvents (Qualigens) were dried and distilled before use by standard methods [20]. Carboxylic acids were used after distillation under reduced pressure (m.p. of myristic acid: 54°C, palmitic acid: 63°C, stearic acid: 70°C and behenic acid: 80°C). Titanium tetrachloride (BDH) was used as received. Titanium was estimated gravimetrically as TiO2 [21].
Physico-chemical measurements
Infrared spectra were recorded on a Perkin Elmer 1600 series FTIR spectrophotometer using KBr discs. 1H and 13C NMR spectra were recorded at 250.17 MHz on a Bruker DPX 250 NMR spectrometer in CDCl3. FAB mass was done on a JEOL SX 102/ DA-6000 mass spectrometer using m-nitrobenzyl alcohol (NBA) as a matrix. Molecular weights were determined in a semi-micro ebulliometer (Gallenkamp) with a thermistor sensing device. Elemental analyses (C, H) were done on a Carlo-Erba 1108 elemental analyzer. Molar conductances were measured on century CC-601 digital conductivity meter at 10-2-10-3 molar solutions in benzene. Solid state conductance measurements were carried out with Keithley 6220 Precision current source and keithley 2182A Nanovoltmeter. Magnetic moment was measured on a Gouy balance using Hg[Co(SCN)4] as a calibrant. Powder XRD data were collected on a PW 1710 BASED diffractometer. The operating voltage of the instrument was 30 kV and the operating current was 20 mA. The intensity data were collected at room temperature over a 2θ range of 5.025 - 79.925° with a continuous scan mode. Transmission electron microscopy (TEM) images were obtained on a Tecnai 30 G2S – Twin electron microscope with an accelerating voltage of 300 kV on the surface of a carbon coated copper grid.
Synthesis of [Ti (OOCC13H27)4] (1)
In myristic acid (2.88 g, 12.61 mmol), a solution of sodium isopropoxide prepared by dissolution of sodium (0.29 g, 12.61 mmol) in isopropanol (10 mL) was slowly added. The contents were refluxed for 2 h. To the sodium salt of myristic acid formed in situ, titanium tetrachloride (0.60 g, 3.16 mmol) in benzene (40 mL) was added dropwise with constant stirring. The mixture was stirred for 1 h followed by refluxing for 4 h. It resulted the formation of sodium chloride which was insoluble in the reaction mixture. This was removed by filtration using G 4 crucible. Excess solvent was removed under reduced pressure. The resulted solid was again dissolved in benzene in which trace amount of sodium chloride left was insoluble. Filtration and drying the solution in vacuo gave a light yellow solid. This gave negative test for chloride ions with silver nitrate. The compound thus obtained was further purified by distillation under reduced pressure (b.p. 220 °C at 5 mm pressure). Complexes 2 to 4 were synthesized analogously which were distilled under reduced pressure at 230 °C, 242 °C, 258 °C respectively and details of analytical as well as spectral results are given in Table 1.
| Compound (Empirical formula) |
Found (Calculated) | IR bands (cm-1) | 1H NMR (δ, ppm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yield (Obtained) | C % | H % | Ti % | Molecular weight | νasymOCO | νsymOCO | Ti–O | ||
| C56H108O8Ti 1 C64H124O8Ti 2 C72H140O8Ti 3 C88H172O8Ti 4 |
2.52 g, 83 % 3.23 g, 88% 3.85 g, 85 % 4.08 g, 81 % |
70.21 (70.24) 71.80 (71.87) 73.18 (73.16) 75.17 (75.14) |
11.41 (11.39) 11.68 (11.71) 11.97 (11.96) 12.28 (12.35) |
4.81 (4.99) 4.45 (4.48) 4.01 (4.05) 3.29 (3.40) |
963 (957) 1055 (1069) 1198 (1182) 1423 (1406) |
1592 1589 1587 1581 |
1467 1465 1462 1458 |
478 476 473 468 |
0.90 [t, 12H; (CH3)4], 1.26 [s, 80H; (-CH2)40], 1.78 [m, 8H; (β-CH2)3], 2.48 [t, 8H; (α-CH2)4] 0.90 [t, 12H; (CH3)4], 1.26 [s, 96H; (-CH2)48], 1.78 [m, 8H; (β-CH2)4], 2.49 [t, 8H; (α-CH2)4] 0.90 [t, 12H; (CH3)4], 1.26 [s, 112H; (-CH2)56], 1.79 [m, 8H; (β -CH2)4], 2.50 [t, 8H; (α-CH2)4] 0.90 [t, 12H; (CH3)4], 1.27 [s, 144H; (-CH2)72], 1.80 [m, 8H; (β -CH2)4], 2.50 [t, 8H; (α-CH2)4] |
Table 1: Analytical and spectral data for titanium tetracarboxylates.
Titanium tetracarboxylates were synthesized by substitutions of chloride ions of titanium tetrachloride by sodium salts of long chain carboxylic acids in 1:4 molar ratio:
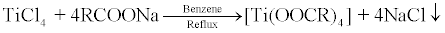
(Where R = C13H27; 1, C15H31; 2, C17H35; 3, C21H43; 4)
All the complexes were soluble in benzene, toluene, chloroform and dichloromethane. The molar conductances (at 10-2-10-3 molar concentrations) in benzene were found 3 to 7 Ω-1cm2 mol-1 which indicated them to be non-electrolytes [22]. Solid state conductance measurements were done for all the complexes and the data were found in the range 1.1×106 - 2.3×106 Ω at 295 K using current 1×10-8 A and voltage 1.4 × 10-2 V. This clearly indicated them to show high resistance and we may say the complexes were behaving as insulators. Magnetic moment measurements indicated the diamagnetic nature for all the complexes which confirmed the absence of unpaired electrons in them. Elemental analyses were in good agreement with the calculated values (Table 1).
Infrared spectra
In infrared spectra of all the complexes O–H stretching vibrations of carboxylic acid (at ~3400 cm-1) were found absent. The bands at 1710 cm-1 (CO stretching) and at 935 cm-1 (OH deformation) of free carboxylic acids were also absent in the spectra. Two strong bands were observed at ~1590 cm-1 and ~1465 cm-1 which could be assigned to (νasymOCO) (antisymmetric) and (νsymOCO) (symmetric) vibrations of the carboxylate ions, respectively. The difference, Δ [νasymOCO–νsymOCO] was ~125 cm-1 which indicated bidentate chelating nature of carboxylate ligands. This resulted in the formation of four symmetrical chelating rings. This enabled us to say that four carboxylate ions gave eight coordination number around Ti(IV) in these carboxylate complexes [23]. Analysis of the infrared bands (Table 1) also revealed that as the length of the alkyl group of carboxylic acids increased, there occurred shifts of νasym(OCO) and νsym(OCO) bands towards lower wave numbers. This effect can be explained by the influence of the type of alkyl group on the strength of the Ti-OOC interactions. The band observed around 470 cm-1 could be assigned to Ti–O vibrations [23].
Hydrolytic stability of the complexes
Titanium(IV) tetracarboxylates exhibited a high hydrolytic stability and were air stable for a longer time. This was tested by dissolving the complexes in benzene followed by adding 1 % water. After stirring the contents in open air for 12 h, no precipitate was apparently visible in the reaction mixture. Excess of solvent was removed in vacuo and no weigh loss was found. The analysis for titanium in this solid indicated to be titanium tetracarboxylate and the composition was not changed during the hydrolysis.
1H NMR spectra
In the 1H NMR spectra of 1–4, no signal for –OH of free carboxylic acids (δ = 10.5 to 12 ppm) were observed indicating deprotonation of the acids. In the spectrum of 1, a triplet appeared at δ = 0.90 ppm (12H) corresponded to methyl protons while a singlet corresponding to 80H of the 40-CH2 groups was observed at δ = 1.26 ppm which could be interpreted for four myristate ions [-OOCCH2CH2(CH2)10CH3] in the complex. The α- and β-CH2 protons of four myristate ions was observed at δ = 2.48 ppm (8H), δ = 1.78 ppm (8H) respectively. Complexes 2, 3 and 4 showed a similar NMR pattern to 1 (Table 1).
13C NMR spectra
The 13C NMR spectra of 1–4 show signals corresponding to the carboxylato ligand. A signal at δ = 38.5 ppm corresponding to the α-carbon atom of methylene group and signals between δ 26.3 to 33.1 ppm corresponding to the carbon atoms of other methylene groups, and for –CH3 carbon, a signal appears at δ = 15.0 ppm. Finally the signal assigned to the carbon atom of –COO group is observed at δ=186.2 ppm.
Both 1H and 13C NMR spectra suggested a similar nature of coordination for all the four carboxylate ions around titanium in the complexes.
Molecular weight and FAB mass
Ebullioscopic method of molecular weight determinations showed that all the complexes were monomeric in refluxing benzene (Table 1). In FAB mass of 1 appearance of a peak at m/z 959 corresponded to its monomeric nature. The peaks at m/z 732, 501 and 274 showed the loss of one myristate ion at each stage. Therefore, at m/z 274 [Ti(OOCC13H27)]3+ unit may be assigned (calculated m/z; 275). Some peaks on lower range may be due to the decomposed ions of indefinite compositions. Almost similar patterns were obtained in FAB mass of complexes 2, 3 and 4.
Powder XRD and TEM Studies
The pattern and results of powder XRD suggested that the complexes showed poor crystallinity. Because of this single crystal XRD could not be done. Powder XRD were done for all the complexes and one spectrum for 2 along with its crystal data is given in Table 2 (Figure 1), which are comparable with titanium oxide oxalate hydroxide hydrate, both in diffraction intensity and position (JCPDS No. 48- 1164). Particle size of the complexes was calculated by the standard Scherrer equation [24].
D = Kλ / (β cosθ)
| Peak No. 2 Theta(°) Flex width d-value Intensity I/Io |
|---|
| 1 13.800 1.176 6.4117 56 9 2 17.800 0.941 4.9789 61 10 3 21.600 0.941 4.1108 635 100 4 24.000 0.941 3.7048 216 34 5 30.000 1.176 2.9761 52 9 6 41.000 -- 2.1995 71 12 7 41.600 -- 2.1692 81 13 |
Table 2: Powder XRD data of complex 2.
Where D is the particle size; K is a constant (= 0.94); λ is X-ray wavelength (λ = 1.5406 Å); θ is Bragg diffraction angle and β is flex width which is converted into radian while calculation. The values obtained were in the range 180-195 nm.
TEM image for the complex 2 is given in Figure 2, which shows the primary particles are spherical in shape having ~200 nm average diameter of the particles.
This communication demonstrates an easy method to synthesize titanium tetracarboxylates which have been isolated as volatile solids and are stable towards air and moisture. The stability and volatility of these metallo-organic titanium(IV) complexes were favoured by achieving a higher coordination number (eight). All the complexes exhibited ideal precursor behaviour and could be a potential candidate for the growth of TiO2 thin film by MOCVD process at higher temperatures. The high solubility of these compounds in common organic solvents makes them suitable for liquid injection MOCVD process. Their physico-chemical characterization made us to conclude the bidentate chelating carboxylate ions around titanium (IV) giving coordination number eight as shown in Figure 3.
Authors are thankful to the CSIR [No. 01(2293)/09/EMR-II] and UGC [No. 37- 132/2009 (SR)], New Delhi for financial supports. They also thank CDRI, Lucknow for spectral and microanalysis.