Case Report - (2024)Volume 9, Issue 1
In recent years, Drug-Induced Liver Injury (DILI) has become a significant concern due to its potential for severe liver damage. We present a case report of autoimmune-like DILI caused by the concomitant use of fenofibrate, atorvastatin and ezetimibe. Glucocorticoid therapy has been proven to be effective. The patient was monitored for an additional 3 years and no changes were observed. Additionally, we provide a comprehensive literature review on similar cases to enhance our understanding of this rare adverse drug reaction. This case highlights the importance of considering autoimmune-like DILI as a potential diagnosis in patients presenting with liver injury and positive autoantibodies. Healthcare professionals should be aware of the potential hepatotoxicity associated with fenofibrate, atorvastatin and ezetimibe. Early recognition and prompt withdrawal of the offending medications, along with appropriate immunosuppressive therapy, can lead to favorable outcomes in patients with autoimmune-like DILI.
Drug-induced liver injury; Autoimmune-like; Fenofibrate; Atorvastatin; Ezetimibe; Case report; Literature review
AIH: Autoimmune Hepatitis; ALP: Alkaline Phosphatase; ALT: Alanine Aminotransferase; ANA: Antinuclear Antibodies; AST: Aspartate Aminotransferase; DBIL: Direct Bilirubin; DI-AIH: Drug-Induced Autoimmune Hepatitis; DILI: Drug-Induced Liver Injury; ESR: Erythrocyte Sedimentation Rate; GGT: Gamma Glutamyl Transferase; MASH: Metabolic Dysfunction-Associated Steato-Hepatiti; RUCAM: Roussel Uclaf Causality Assessment Method; TC: Total Cholesterol; TG: Triglyceride; TBIL: Total Bilirubin; ULN: Upper Limit Of Normal; CT: Computed Tomography; MRCP: Magnetic Resonance Cholangio Pancreatography; AIH: Autoimmune Hepatitis; MRI: Magnetic Resonance Imaging; RDC: Revised Diagnostic Criteria; SDC: Simplified Diagnostic Criteria; IgG: Immunoglobulin G; NASH: Non-Alcoholic Steatohepatitis; iAIH: idiopathic Autoimmune Diseases
Fenofibrate, atorvastatin and ezetimibe are commonly used agents for managing dyslipidemia and reducing cardiovascular risk. However, hepatotoxicity is a concerning side effect associated with their use. While mild and asymptomatic elevation of aminotransferases (Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT)) is common, significant elevation of these enzymes is rare, occurring in less than 3% of cases [1,2]. However, there have also been reports of severe jaundice caused by fenofibrate in clinical practice [3]. Cases of liver Injury caused by statins are slightly more common. Non-specific autoantibodies, clinical and laboratory features of an autoimmune-like hepatitis may be present [4]. Ezetimibe is metabolized by the P450 system, but has little effect on the pharmacokinetics of other drugs, common side effects of ezetimibe include diarrhea and flatulence. Rare, potentially severe adverse effects include hypersensitivity reactions, myopathy and rhabdomyolysis. Clinically apparent serious liver injury due to ezetimibe has been reported, but is rare. Cases of autoimmune hepatitis-like injury have been described in patients taking the combination of ezetimibe. Furthermore, because this agent is often used in combination with other cholesterol lowering drugs, the role of ezetimibe in these reports is not always well defined [5].
In this report, we present a case of drug-induced liver injury associated with fenofibrate. Subsequent treatment with atorvastatin or ezetimibe resulted in abnormal liver function and autoimmune characteristics in the patient. Glucocorticoid treatment was administered and during long-term follow-up, transaminase levels remained stable without further elevation after gradual reduction of glucocorticoid dosage. The patient was monitored for an additional 3 years and no changes were observed.
The patient is a 63-year-old female, Height (Ht): 155 cm, Weight (Wt): 46.8 kg, Body Mass Index (BMI): 19.5 kg/m2. Due to hypertriglyceridemia after diet control and lifestyle adjustment still did not reach the standard: Triglycerides (TG) 7.36 mmol/L. The cardiovascular doctor prescription taking Fenofibrate treatment 200 mg/day. There are no abnormalities in basic liver function and hepatitis A, B, C, D and E. After 15 days of medication, slight abnormalities in serum aminotransferase levels were observed. After continuing medication for one month, liver function was abnormal: (ALT 236 U/L↑, AST 150 U/L↑, GGT 65 U/L↑ and ALP 86 U/L↑), TBIL was still normal. TG decreased significantly, but the serum aminotransferase levels increased, considering drug- induced liver injury. Immediately stopped fenofibrate treatment, four months later, liver function was normal and maintained for two years. Later, because the patient was worried about hyperlipidemia, try Atorvastatin 20 mg/day, one month later, there was a slight abnormality in transaminase levels. After two months of drug medication, we have once again discovered abnormalities in liver function (ALT 200 U/L↑, AST 187 U/L↑, GGT 231 U/ L↑ and ALP 207 U/L↑) and TBIL was normal. The patient has no clinical symptoms and stopped taking the medication again. After taking polyene phosphatidyl choline orally for one month, the transaminase level continued to rise. At the time of discontinuation for two months, more severe abnormalities in liver function (ALT 279 U/L↑, AST 456 U/L↑, GGT 453 U/L↑ and ALP 272 U/ L↑), TBIL was normal and albumin was 34.1 g/L. The patient did not drink alcohol, did not take antipyretic and analgesic drugs or herbal medicines. Physical examination showed no signs of chronic liver disease. Complete examinations were conducted with ESR 53 mm/h↑, ANA 1:1000↑, anti LKM1 antibody (-), anti-Solid Liver Antigen (anti SLA) antibody (-) and IgG 17.13 g/L↑ (7-16 g/L). Blood routine, coagulation function and all hepatitis virus tests are negative. No relevant abnormalities were found in liver ultrasound, Computed Tomography (CT) and Magnetic Resonance Cholangio Pancreatography (MRCP). We excluded viral hepatitis, alcoholic hepatitis, hereditary liver disease and fatty liver disease. According to LiverTox [6], considering liver injury caused by atorvastatin, the Roussel Uclaf Causality Assessment Method (RUCAM) score is 7 (likely). However, the patient's liver function did not recover after discontinuing the medication and at the same time, it showed positive autoantibodies and elevated immunoglobulin IgG. Based on the 2018 AIH scoring diagnostic system, the patient's score was 12 points, indicating the presence of drug-induced autoimmune hepatitis Drug-Induced Autoimmune Hepatitis (DI-AIH) or Autoimmune Hepatitis (AIH) [7]. In order to further distinguish, we made liver biopsy, inflammation is mainly present in the lobules of liver tissue, with significant liver cell swelling and mixed inflammatory cell infiltration (visible in eosinophils, neutrophils and lymphocytes), apoptotic bodies are visible and inflammation in the portal area is not significant (Figures 1 and 2).
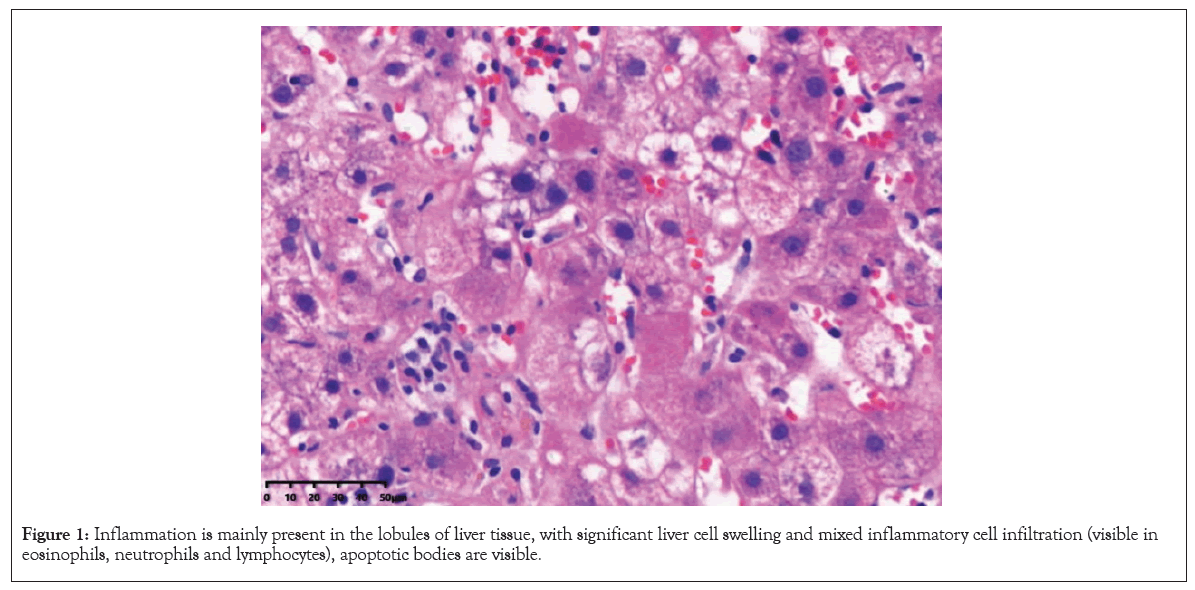
Figure 1: Inflammation is mainly present in the lobules of liver tissue, with significant liver cell swelling and mixed inflammatory cell infiltration (visible in eosinophils, neutrophils and lymphocytes), apoptotic bodies are visible.
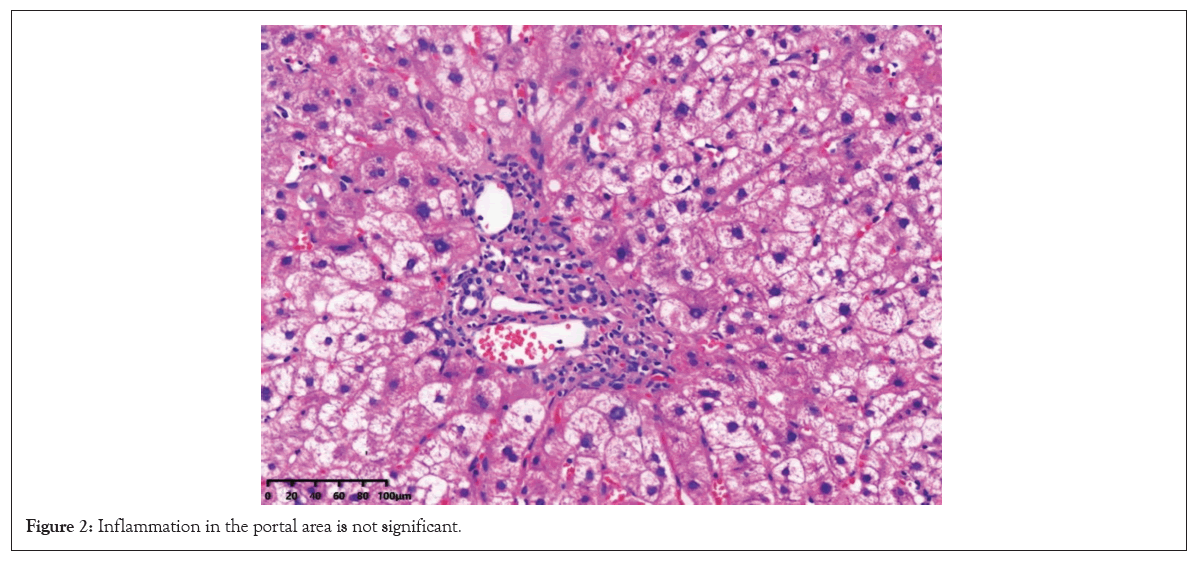
Figure 2: Inflammation in the portal area is not significant.
There is no clear pathological evidence of AIH in the presentation of acute liver injury. We administered standard doses of polyene phosphatidylcholine and ursodeoxycholic acid for treatment, with methylprednisolone tablets at a dose of 24 mg/day gradually reduced. After 3 months, the patient's liver function was all normal, with normal IgG and erythrocyte sedimentation rate. After stopping the medication for 5 months, the patient's methylprednisolone dosage was reduced to 4 mg/day and liver function remained normal upon reexamination. The patient attempted to choose ezetimibe 10 mg/day for lipid-lowering and liver function remained normal in the first month. However, at 3 months, the patient once again experienced elevated transaminases (ALT 788 U/L↑, AST 554 U/L↑, GGT 297 U/L↑ and ALP 179 U/L↑), TBIL was normal, ANA 1:100. Liver Magnetic Resonance Imaging (MRI): Chronic liver disease. Stop the medication again and administer magnesium Isoglycyrrhizinate injection and acetylcysteine injection for treatment. We did not adjust the dosage of methylprednisolone and still maintained it at 4 mg/day. Liver enzymes has decreased, after 5 months, liver enzymes and IgG were all normal. Due to the patient's comorbidities of dry syndrome and autoimmune thyroiditis, the patient has been receiving oral methylprednisolone 2 mg/day for maintenance treatment. Currently, liver function has remained normal for three years (Figure 3 and Table 1).
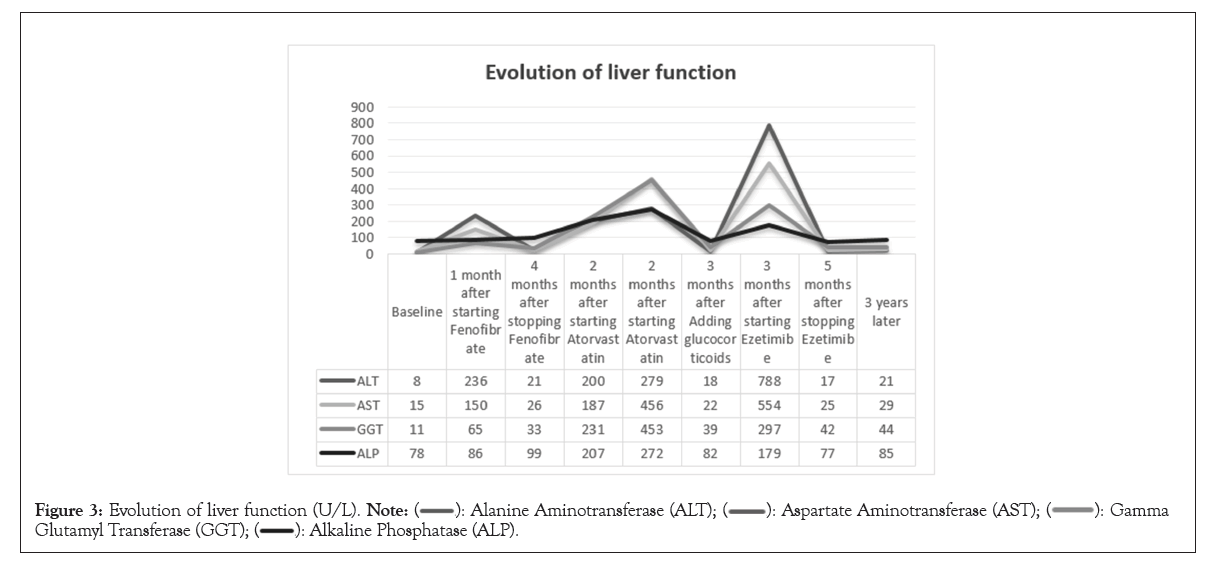
Figure 3: Evolution of liver function (U/L). 

| Baseline | 1 month after starting Fenofibrate | 4 months after stopping Fenofibrate | 2 months after starting Atorvastatin | 2 months after stopping Atorvastatin | 3 months after adding glucocorticoids | 3 months after starting Ezetimibe | 5 months after stopping Ezetimibe | 3 years later | |
|---|---|---|---|---|---|---|---|---|---|
| ATL (7-40U/L) | 8 | 236 | 21 | 200 | 279 | 18 | 788 | 17 | 21 |
| AST (13-35)U/L | 15 | 150 | 26 | 187 | 456 | 22 | 554 | 25 | 29 |
| GGT (7-45 U/L) | 11 | 65 | 33 | 231 | 453 | 39 | 297 | 42 | 44 |
| ALP (35-100 U/L) | 78 | 86 | 99 | 207 | 272 | 82 | 179 | 77 | 85 |
| TBIL (3.4-20.5 umol/L) | 18.2 | 10.8 | 11.8 | 10.3 | 16.2 | 10.6 | 13.8 | 14.9 | 10.9 |
Note: ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; GGT: Gamma-Glutamyl Transferase; ALP: Alkaline Phosphatase; TBIL: Total Bilirubin.
Table 1: Changes in liver function.
Drug induced liver injury is an important adverse drug reaction, which has been proven to be caused by over 1000 drugs, herbs or dietary additives [8]. DILI can manifest as acute or chronic liver disease, which can be divided into liver cell injury type, cholestasis type and mixed type according to the type of liver injury. Most cases are self-limiting after discontinuation of medication, but it may also lead to serious consequences such as chronic liver injury, liver transplantation or death [9-15]. The diagnostic criteria for acute DILI are (i) ALT ≥ 5ULN; (ii) ALP ≥ 2ULN; (iii) ALT ≥ 3ULN and (iv) TBil ≥ 2ULN. The histological manifestations of DILI are diverse, covering almost all types of liver pathological changes. The gold standard for DILI diagnosis is that taking the same medication again causes the same liver damage, but attempting again poses a fatal risk to the patient. Due to the lack of specific diagnostic markers, the commonly used method for determining the causal relationship between drugs and liver injury is the Roussel Uclaf Causality Assessment Method (RUCAM), which includes two assessment forms: liver cell injury type and cholestasis type/mixed type. Diagnosis relies on a comprehensive assessment of medical history, clinical symptoms, serum biochemistry, immunology and histology and is an exclusive diagnosis [16].
Fenofibrate is a fibrous acid derivative used to treat hypertriglyceridemia and dyslipidemia. Among patients receiving fenofibrate treatment, up to 20% of patients experience mild and transient elevation of serum transaminases, only 3%-5% of patients experience an elevation three times higher than normal. These abnormalities are usually asymptomatic and transient and can be resolved even with continued use of fenofibrate. However, occasional significant discomfort symptoms and elevated serum transaminases require discontinuation of medication [17]. But there are also literature reports on cases of liver injury that occur immediately after 48 hours of taking fenofibrate, as well as cases of severe liver injury caused by fenofibrate [3,18]. Autoimmune mediated liver injury has not yet been found. The patient we reported first used fenofibrate and developed obvious liver injury, consistent with drug-induced liver injury. After discontinuation for a period of time, liver function returned to normal. Therefore, we believe that there is a causal relationship between liver dysfunction and the use of fenofibrate.
The patient subsequently experienced liver dysfunction again after using atorvastatin during normal liver function. Atorvastatin is the main rate limiting enzyme in cholesterol synthesis. 1%-3% of patients may experience mild, asymptomatic transient elevation of serum transaminases, but in less than 1% of patients, serum transaminase levels are three times higher than ULN and most of the increases are self-limiting and do not require dose adjustment.
Atorvastatin hepatotoxicity can also manifest as a clear pattern of liver cell damage, but at least one-third of liver cell cases have autoimmune features, characterized by high levels of immunoglobulin, positive ANA and liver biopsy results of autoimmune hepatitis, indicating that it may be immune mediated. DI-AIH has been reported to occur in monotherapy with statin drugs such as simvastatin, rosuvastatin and atorvastatin or in combination with other lipid- lowering drugs [19-21]. Either a positive ANA or a smooth muscle antibody is found in approximately two out of three patients with statin-associated DI-AIH [21]. Yehia Saleh, et al., [22] reported a case of atorvastatin induced autoimmune myopathy and glucocorticoid therapy is effective, further suggesting the possibility of atorvastatin inducing autoimmune diseases. Related studies have found that in some patients with atorvastatin induced DI-AIH, more patients relapse after glucocorticoid discontinuation, indicating that atorvastatin is more likely to induce classical AIH [23]. The patient we reported experienced liver injury again after using atorvastatin during normal liver function and exhibited autoimmune features such as high immunoglobulin levels and positive ANA. After treatment with glucocorticoid, the liver function has been restored. We believe that atorvastatin caused liver injury and exhibited autoimmune mediated characteristics.
After two liver injuries, the patient voluntarily chose different mechanisms of action of ezetimibe to lower blood lipids, which also resulted in liver injury and exhibited autoimmune characteristics again. Ezetimibe is a type of intestinal cholesterol absorption inhibitor with a unique mechanism of action and chemical structure. There is no reason to doubt the cross sensitivity of ezetimibe to liver injury with other cholesterol lowering drugs. Compared with statin therapy, severe side effects are very rare, particularly when the drug is used as monotherapy in patients with statin intolerance [24]. In fact, a lot of previous published cases of ezetimibe-induced liver injury have been reported in combination with a statin [25,26]. Reports of severe acute liver injury caused by the sole use of ezetimibe are very rare. However, cases of autoimmune hepatitis like syndrome suggest that immune mediated damage may be the cause of some clinically significant liver damage caused by ezetimibe.
Autoimmune Hepatitis (AIH) is a severe liver disease The diagnosis of AIH depends on increased serum transaminase and immunoglobulin G levels, presence of autoimmune and interface diseases on liver history. The International Autoimmune Hepatitis Group published Revised Diagnostic Criteria (RDC) for AIH in 1999 and Simplified Diagnostic Criteria (SDC) in 2008. A pretreatment RDC score of 15 is considered defined for the diagnosis of AIH, a pretreatment RDC score of 10 notes a possible diagnosis of AIH [7]. Our patient scores 12.
Autoimmune like drug induced liver injury is a subtype of DILI, which has been increasingly recognized and reported in recent years. Drugs that cause immunity related DILI can trigger immune response against hepatic proteins which can lead to a clinical course similar to autoimmune diseases DI-AIH and DILI are both exclusive diagnoses. Similar to DILI, the incubation period of most DI-AIH is greater than 2 months, the pattern of life injury with DI-AIH is typically heterocellular with 'R' ratio>5, Alanine Aminotransferase (ALT) compared to Alkaline Phosphatase (ALP) [9]. The appearance of higher levels of Immunoglobulin G (IgG) tends to favor DI-AIH, a positive Antinuclear Antibodies (ANA), although non-specific, is found in anywhere from 50% to 83% of patients with DI-AIH [27-29]. Anti-smooth drug antibiotics are less frequently positive in about 55% of cases. Due to the high levels of immunoglobulin and positive ANA in the patient's serum biochemistry results, in order to further distinguish whether the patient is DI-AIH or AIH, our case improved liver biopsy histology, which showed acute liver injury. No typical histological manifestations of AIH were observed.
DI-AIH is a challenging diagnosis. Although autoantibodies are common, they are neither specific nor diagnostic for DI-AIH and may appear in other non-immune mediated liver diseases, including Non-Alcoholic Steato-Hepatitis (NASH). Histology may help describe the subtle differences between DI-AIH and newly developed iAIH, but the two are often indistinguishable histologically. Although RUCAM is a useful tool, any drug should consider DI-AIH if there are features that suggest autoimmunity. The complete recovery of liver injury is most common in DI-AIH, Long term injury may also occur and immunosuppressive therapy may be needed. If there is a suspected recurrence of DI-AIH after discontinuing corticosteroids, the possibility of iAIH should be considered [30,31].
Based on the above, continuing to observe whether the disease has recurred or conducting another liver biopsy, will be helpful in making a clear diagnosis of DI-AIH or AIH.
In order to detect liver Injury earlier, we suggest that hepatic enzymes must be measured at least one month or even earlier after initial hepatic drug treatment.
The reason why it is difficult to distinguish between AIH and DILI is because their clinical manifestations are similar, AIH autoantibodies do not have specificity and DILI also lacks highly reliable diagnostic criteria. In general, the diagnosis of AIH requires excluding the influence of medication and a history of medication happens to be a prerequisite for diagnosing DILI. However, the relationship between DILI and drugs is not significant and individual differences are significant. It is extremely difficult to clarify the causal relationship between drugs and liver injury. On the other hand, there are still drug-mediated DI-AIH in clinical practice, so there are certain limitations in distinguishing DILI, DI-AIH and AIH based on drug history. According to literature reports, autoantibodies are neither specific nor diagnostic of DI- AIH, advanced age and female gender appearance to prepare one to DI-AIH, liver biopsy may be helpful in describing sub differences between DI-AIH and de novo idiopathic Autoimmune Hepatitis (iAIH).
For first-time onset, clear medication history, obvious autoimmune characteristics, glucocorticoid therapy has been proven effective after discontinuing suspicious drugs. After the condition improves, the dosage gradually decreases until the medication is stopped, if there are no signs of recurrence during the follow-up process, DI-AIH diagnosis is supported. If the condition relapses without further medication, it can be diagnosed as AIH.
This case highlights the importance of considering DI-AIH as a potential diagnosis in patients presenting with liver injury and positive autoantibodies. Healthcare professionals should be aware of the potential hepatotoxicity associated with fenofibrate, atorvastatin and ezetimibe. Early recognition and prompt withdrawal of the offending medications, along with appropriate immunosuppressive therapy, can lead to favorable outcomes in patients with DI-AIH.
The institutional review boards of Hangzhou Third People’s Hospital approved this retrospective study and the requirement for written informed consent was waived due to its retrospective study nature.
Written informed consent for publication has been obtained from the participants in this study.
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
All data generated or analyzed during this study are included in this published article.
All the authors have no conflicts of interests to declare.
Not applicable
Yahong He and Jiabin Xiong collected the data. Kejie Hu analyzed the data. Kejie Hu and Yufang Wang wrote the manuscript. Yufang Wang prepared all tables and figures. All authors read and approved the final manuscript.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Hu K, Wang Y, He Y, Xiong J (2024) Autoimmune-Like Drug-Induced Liver Injury caused by Fenofibrate, Atorvastatin and Ezetimibe: A Case Report. Immunogenet Open Access. 9:222.
Received: 01-Mar-2024, Manuscript No. IGOA-24-30087; Editor assigned: 04-Mar-2024, Pre QC No. IGOA-24-30087 (PQ); Reviewed: 19-Mar-2024, QC No. IGOA-24-30087; Revised: 26-Mar-2024, Manuscript No. IGOA-24-30087 (R); Published: 02-Apr-2024 , DOI: 10.35248/IGOA.24.9.222
Copyright: © 2024 Hu K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.