Rheumatology: Current Research
Open Access
ISSN: 2161-1149 (Printed)
ISSN: 2161-1149 (Printed)
Research - (2019)Volume 9, Issue 1
Autoimmune Rheumatic Diseases (ARD) have been associated with Silicon Breast Implant (SBI) as part of the spectrum of Autoimmune/Inflammatory Syndrome Induced by Adjuvants (ASIA). Silicone is an adjuvant that may bleed and subsequently may be a chronic stimulus to the immune system.
Objective: To determine the prevalence of autoimmune diseases (AID) and risk factors associated to patients with ASIA induced by SBI (ASIA-SBI).
Patients and methods: This study was performed between 2012 and 2018, in a tertiary referral center, from a cohort of 210 patients with ASIA. We selected those patients with ASIA-SBI and clinical manifestations of any ARD. We investigated family history of AID, smoking, allergies and comorbidities. Statistical analysis: Chi square and Odds Ratio (OR).
Results: There were 45 women with current age 50 (29-75) years, mean time of appearance of clinical manifestations after SBI was 8.8 ± 5.5 years. We found systemic sclerosis (SSc) 10 patients, rheumatoid arthritis (RA) 8, undifferentiated connective tissue syndrome (UCTDS) 6, fibromyalgia (FM) 5, systemic lupus erythematosus (SLE) 4, Sjogren syndrome (SS) 3, angioedema/urticaria 3, overlap syndrome 2, and one of each of the following: Takayasu arteritis, Still disease, tunnel carpal syndrome and antiphospholipid syndrome. We observed family history of ARD 42%, allergy history 37.5% and smoking 35.5%. In ASIA-SBI patients, family history and smoking had a significant association with SSc OR 6.0 (CI 1.2-29), p<0.02 and RA OR 7.1 (CI 1.3-37.2), p<0.022.
Conclusions: In patients with ASIA-SBI, family history of AID and smoking were associated with SSc and RA. In addition to SBI, environmental and genetic factors should be taken into account for the development of AID.
Autoimmune/Inflammatory syndrome induced by adjuvants (ASIA); Silicone breast implant (SBI); Autoimmune rheumatic disease (ARD); Systemic sclerosis (SSc); Rheumatoid arthritis (RA); Systemic lupus erythematosus SLE); Undifferentiated connective tissue syndrome (UCTD) and risk factors
Autoimmune/Inflammatory Syndrome Induced by Adjuvants (ASIA) is a new syndrome which includes the following: siliconosis, Gulf War syndrome, macrophagic myofasciitis syndrome, and post-vaccination phenomena. Siliconosis comprises a broad spectrum of clinical entities, including Silicone Breast Implant (ASIA-SBI) [1,2]. In epidemiological studies, the association of SBI and autoimmune diseases (AID) has been controversial [3,4]; however some studies, support the association between SBI and AID [5,6]. Recently, a study that included a large number of patients (99,993) with SBI, found a significant association between SBI and diseases such as Sjogren syndrome (SS), systemic sclerosis (SSc), and rheumatoid arthritis (RA) [7]. In this regard, it has been reported that silicones may develop oxidization to silica and also silicon-containing gel induces an increase of immune response activity [8]. The mechanism through which silicones produce autoimmunity is similar to the model proposed for other adjuvants by the activation of innate and adaptive immunity and activation of the Th1 and Th17 response with release of interleukin 17 that stimulates fibroblasts leading to fibrosis [9,10]. Therefore, disorders in the modulation of cytokines can be responsible in susceptible individuals, for a perpetuation of the inflammatory response which can locally lead to capsule contracture and at the systemic level to possible triggering of AID [11]. Hence, silicone present in the breast prosthesis acts as an adjuvant [8,12]. However, the presence of other environmental factors associated with SBI-AID has been scarcely studied.
Patients and methods
This study was performed in a tertiary referral center from 2012 to 2018, from a cohort of 210 patients with ASIA according to Shoenfeld´s criteria [1]. The inclusion criteria were: patients with SBI, clinical manifestations of any AID and criteria for ASIA. Exclusion criteria: patients with SBI plus other adjuvants such as vaccines, mineral oil and other modelant substances, patients with rheumatic disease prior to SBI.
We retrospectively analyzed files of patients that met criteria for ASIA- SBI. This study was approved by local Committee of the Hospital. In our ASIA clinic, all patients are investigated for ARD clinical manifestations and autoantibodies are determined.
Clinical manifestations of ARD were evaluated and classified according to established criteria of The American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) for RA, SLE, SSc, SS, inflammatory myopathies, vasculitis, antiphospholipid syndrome (APS). We also investigated overlap connective tissue disease syndrome (OCTDS), undifferentiated connective tissue syndrome (UCTDS); other rheumatic diseases such as fibromyalgia (FM), and diseases of allergic origin i.e. angioneurotic edema (AE) and urticaria.
We also evaluated personal and family history of AID, tobacco smoking, coexisting allergies and comorbidities such as autoimmune hypothyroidism, depression, anxiety and others.
We also evaluated the following: antinuclear antibodies (ANA) determined by indirect immunofluorescence, with normal values less than 1:80, antibodies to double stranded DNA (Anti sdDNA), anti-topoisomerase I (formerly called Scl-70), anticentromere (ACA), Sjogren’s syndrome antibodies-A (SSA) or anti- Ro, Sjogren ’ s syndrome antibodies-B (SSB) or anti-La, rheumatoid factor (RF), anticyclic citrullinated peptide (ACPA), anti-cardiolipin antibodies (aCL), lupus anticoagulant (LAC) and thyroid peroxidase antibodies (TPO). We also measured Creactive protein (C-RP), erythrocyte sedimentation rate (ESR), cell blood count, liver function, and urine. Histological studies (lymph node biopsy, breast prosthesis and skin) and imaging studies (Magnetic Resonance Imaging (MRI), computed tomography (CT), ultrasound of breasts and mammography) were undertaken for determining affection of SBI. We also analyzed medical and surgical treatments used in these patients.
Statistical analysis
Descriptive statistics was used to summarize the baseline demographic and clinical characteristics of ASIA-SBI. Continuous variables were presented as a mean ± standard deviation (SD) value, and discrete variables were reported as median (minimum-maximum). We used chi square and Odds Ratio (OR) with confidence intervals at 95% for the analysis of ASIA-SBI and risk factor for development of AID.
We studied 45 female patients with ASIA-SBI (all patients received different types of implants from different manufacturers for cosmetic purposes). One of these patients had additionally implants of silicon in buttocks and in right eye. The median current age was 50 (29-75) years and the age at placement of the prostheses was 30 (15-43) years. The mean disease course is 11 ± 4.9 years. Table 1 shows demographic data, associated factors and comorbidities. Family history of AID and tobacco smoking were statistically significant risk factors for the development of systemic sclerosis and rheumatoid arthritis. The most frequent comorbidities were hypothyroidism of autoimmune origin and depression.
| Different type of cases | Number | Percentage (%) |
|---|---|---|
| Sex-Female | 45 | 100 (%) |
| Current age (Years) | -- | -- |
| Median (Range) | 50 | (29-75) |
| Age at placement SBI | -- | -- |
| Median (Range) | 30 | (15-43) |
| Mean time from onset of symptoms (Years) | 8.8 ± 5.5 | -- |
| Mean disease course of disease. | 11 ± 4.9 | -- |
| Family history of rheumatic disease * | 19 | 42% |
| Allergies | 17 | 37.5 |
| Tobacco smoking * | 16 | 35.5 |
| Deaths | 1 | 2.2 |
| Comorbidities | -- | -- |
| Autoimmune hypothyrodism | 10 | 22 |
| Depression | 8 | 17.7 |
| Systemic arterial hypertension | 7 | 15.5 |
| Obesity | 5 | 11.1 |
| Asthma | 2 | 4.4 |
| Neuropathies | 1 | 2.2 |
| Thyroid cancer | 1 | 2.2 |
| Infertility (Low levels of anti-mullerian hormone) | 1 | 2.2 |
| Infertility and pituitary adenoma | 1 | 2.2 |
* A Chi-square analysis showed a significant association between family history and tobacco smoking with an increased risk for systemic sclerosis: OR 6.0 (CI 1.2-29), p<0.02 and rheumatoid arthritis: OR 7.1 (CI 1.3-37.2), p<0.022.
Table 1: Demographic data, associated factors and comorbidities in patients with ASIA-silicone breast implant.
Table 2 shows patients with SBI who met criteria for some AID. SSc was the most frequent, followed by RA, UCTD and SLE. Regarding rheumatic diseases, FM was found in 5 patients (11%), without an association to rheumatic diseases; in addition we found FM in ASIA-SBI associated to other rheumatic diseases in another 5 patients. We also observed three patients with angioneurotic edema (AE), one patient with Takayasu arteritis, and one with adult onset Still's disease (AOSD), among others.
| Autoimmune rheumatic diseases | ||
|---|---|---|
| Rheumatic disease | Patients | Percentage (%) |
| Systemic sclerosis | 10 | 22 |
| Rheumatoid arthritis | 8 | 17.7 |
| Undifferentiated connective tissue diseases | 6 | 13.3 |
| Systemic lupus erythematosus | 4 | 8.8 |
| Sjogren syndrome | 3 | 6.6 |
| Overlap syndrome | 2 | 4.4 |
| Vasculitis: Takayasu arteritis | 1 | 2.2 |
| Adult Still's disease | 1 | 2.2 |
| Antiphosfolipid syndrome | 1 | 2.2 |
| Allergies and rheumatic disease | -- | -- |
| Angioneurotic edema/urticaria | 3 | 6.6 |
| Fibromyalgia (FM) * | 5 | 11.1 |
| Carpal tunnel syndrome | 1 | 2.2 |
*Fibromyalgia associated to ASIA- SBI was found in 5 patients. Fibromyalgia associated to other AID was found in other 5 patients
Table 2: Autoimmune disease in patients with ASIA-silicon breast implant. N=45.
The principal immunological laboratory findings were ANA with low to medium levels in the majority of cases, although in some cases they were high. In all SSc patients there were more ACE than anti Scl-70. All patients with RA had positive RF and SLE patients showed ANA with homogeneous pattern or diffuse patterns, as well as anti dsDNA, and anti RNP. Patients with ASIA – SBI and FM had low levels of ANA and other autoantibodies such as anti Ro, anti La and anti RNP. The range of numbers of ANA was from 1 to 5. We also noted increased CRP and ESR (Table 3).
| Autoantibody | Number of patients | Percentage (%) |
|---|---|---|
| Anti-nuclear antibodies | 37 | 82.2 |
| Anti-centromere antibodies (ACE) | 10 | 22.2 |
| Anti SCl 70 | 4 | 8.8 |
| Anti Ro/La | 3 | 6.6 |
| Anti RNP | 6 | 13.3 |
| Anti-cardiolipin antibodies | 1 | 2.2 |
| Anti - Factor VIII adquired | 1 | 2.2 |
| Anti-cyclic citrullinated peptide (ACPA) | 7 | 15.5 |
| Rheumatoid factor | 8 | 17.7 |
| C-reactive protein increased | 33 | 73.3 |
| Erythrosedimentation rate increased | 31 | 68 |
| Complement C3/C4 levels low | 3 | 7.6 |
| Silicone Breast Implant N=45 | ||
Silicone Breast Implant N=45
Table 3: Laboratory findings in patients with ASIA-Silicone breast implant. N=45.
The most common adverse reactions after silicone breast implant (SBI) were capsular contracture, rupture and infection (Figure 1). The main histological findings were chronic granulomatous inflammation and body type reactions from implant fibrous capsule to silicone corpuscles (Figure 2).
Figure 1. Silicone breast implant, A-contracture of silicone breast implant grade III, B-contracture of silicone breast implant grade IV and rupture with chronic presence of silicone (like-caseous material).
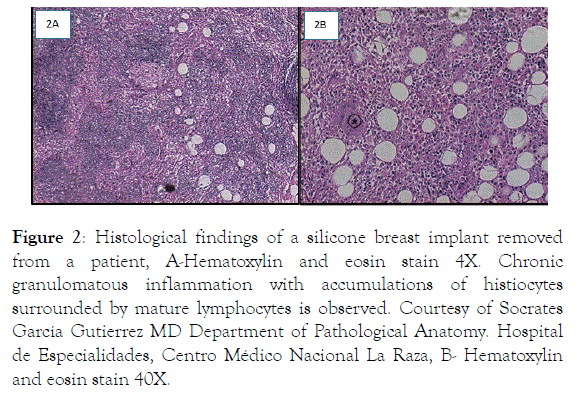
Figure 2. Histological findings of a silicone breast implant removed from a patient, A-Hematoxylin and eosin stain 4X. Chronic granulomatous inflammation with accumulations of histiocytes surrounded by mature lymphocytes is observed. Courtesy of Socrates García Gutierrez MD Department of Pathological Anatomy. Hospital de Especialidades, Centro Médico Nacional La Raza, B- Hematoxylin and eosin stain 40X.
Regarding treatment, all patients were managed according to main rheumatic disease. Patients with SSc received methotrexate (MTX), mycophenolate mophetil, calcium channel blockers, proton pump inhibitor, and non-steroids anti-inflammatory drugs (NSAIDs). Patients with RA received MTX and low doses of prednisone during short periods and flares. Patients with SLE received chloroquine, prednisone and one case of neuropsychiatric lupus received methylprednisolone pulses and bimonthly cyclophosphamide over a year. UCTDS patients were treated with steroids or NSAIDs and patients with FM were treated with antidepressants (inhibitors of serotonin recapture), NSAIDs and psychological therapy. All of the above treatments provided complete response in some patients or incomplete control with remissions and exacerbations of AID.
Prosthesis removal was done in 16 patients due to shrinking and/or breakage, with manifestations of pain. In addition, two patients had infections, with re-implantation of new prosthesis in 8 patients, with improvement of symptoms in 5.
We evaluated 45 patients with ASIA-SBI that developed various ARD, being SSc, RA, UCTD and SLE the most frequent, followed by SS, OCTD, AE, AODS and APS. In ASIA-SBI patients, family history and smoking had a significant association for developing of SSc and RA.
These ARD especially SSc are more frequent than those observed in others studies [2,13]. The majority of these patients had family history of ARD such as RA (2 patients), granulomatosis with polyangiitis (one patient), and SLE (3 patients). One patient had asthma and two patients were allergic to drugs. None of them had history of working exposure to silicon. Several causative agents have been proposed as etiology of SSc, chemicals have been the most suggested ones. Among them, silica and SBI have been related to SSc development; however, this association has been controversial. Different metaanalyses did not find an increased risk of the association of SBI and SSc, yet, these meta-analyses have some biases [14,15]. Rubio Rivas et al. [16] found an increased risk of SBI to induce SSc. Recently in a large post approval study a significant association between SBI and AID such as SSc, SS and RA was found [7]. However, in several studies, some confounding factors were not taken into account, such as lifestyle, smoking, exposure to ultraviolet light, history of cancer and family history among others, which are important in the development of AID [7,17].
In our patients, the average time from placement of SBI to first manifestations of SSc was greater than 8 years, which indicates a long period for the appearance of SSc. Our patients also had a family history of AID which implies a close interaction between environmental factors, genetic factors and the immune system.
The second ARD was RA, this disease was observed in 8 patients, 4 patients had family history of ARD and 4 patients had history of smokingThe association of working exposure and AID is well recognized for silica, and suspected for others chemical substances. Among heavy smokers, both silica and other inorganic dust exposures have been associated with increased risk of RA [18]. In addition to the family history for RA, environmental factors such as smoking have been associated with triggering of RA, however, there are other candidates such as silicone [19,20]. Silicon and SBI may cause autoimmune responses which lead to the development of RA [2,21,22]. Recently a strong association between SBI and RA with a Standardized incidence ratio (SIR) of 5.96 was found [7].
In the present study, a case of AOSD also was associated with SBI. In our patient there was a previous history of probable systemic onset juvenile idiopathic arthritis, who in adolescence received SBI and later developed AOSD. With regard to this disease there are some case reports attributed to SBI [23,24].
With respect to SLE, we found 4 cases, with a broad spectrum of autoantibodies, including antibodies against factor VIII and manifestations of bleeding with diagnosis of acquired hemophilia plus SLE in one patient. Another patient had severe neuro-psychiatric manifestations. The other two patients had hematological SLE and cutaneous and articular conditions. One patient had history of heavy smoking and another of family history of ARD. In experimental models of murine lupus, it has been demonstrated that implantation of silicone influences the immune response and the production of different autoantibodies [25]. It has been reported that some of the patients with SBI developed clinical manifestations that did not meet established criteria for SLE, but had various self-antibodies as anti-Ro [26]. Other studies have reported the association of SBI and SLE, as well as the generation of various autoantibodies [27].
With regard to SS, an epidemiological study investigated the risk of ARD and SBI patients finding a relative risk of 2.0 [28]. Recently, it was reported that SS had a significant association with SBI with a SIR of 8.14 [7].
We had 2 cases with overlap syndrome (one had SSc, RA and SS and the other, RA and SSc), this association has also been reported in cases with SBI [29,30]. On the other hand, several cases or series of cases have shown that patients with SBI met criteria for ASIA and also for non-specific manifestations of definite diseases of connective tissue and presence of ANA, entity which can be termed UCTD [30-32]. The majority of these patients had comorbidities, such as autoimmune hypothyroidism and one case had infertility with low levels of anti mullerian hormone not associated with the use of immunosuppressants. In relation to other AID, we found a case of Takayasu arteritis with clinical manifestation of fever of unknown origin, adenopathies and lack of pulses; there are no prior reports of association with SBI in the literature. Some studies have only reported systemic vasculitides associated to anti-neutrophil cytoplasmic antibodies (ANCA) and SBI [2].
With respect to rheumatic diseases, we found 5 patients with FM, who had non-specific manifestations of connective tissue or UCTD. In addition, we also found another 5 cases of secondary FM associated with some AID. In these cases there is an overlap of manifestations being pain, fatigue and neurological disorders (cognitive alterations) the most prevalent. Recently Khoo [33] reported 30 patients with SBI in whom 10% had FM; we found a higher frequency in our study. FM shares some manifestations with ASIA criteria. Factors of atopy and hyperimmune state as well as low levels 5-Hydroxyindoleacetic acid (5-HIAA) a serotonin metabolite have related to ASIA and FM respectively [34]. Other factors have also been implicated, such as surgery for exchange of SBI. Hence, SBI can be considered as trigger of FM [34]. Further, SBI surgery and depression can be stressful events for the patient and can be considered risk factors for suicide [33,34]. In this context, we observed one suicide in our study. We found criteria for mild to moderate depression in 6 patients and for severe in 2.
Another disease observed was angioneurotic edema where there was a history of allergy to drugs and food in all patients. This entity has already been reported in patients with SBI [29].
Finally, we consider that there are patients who are not candidates for SBI placement, such as patients with family history of AID and allergies or atopic patients as previously described [30]. We suggest that before these patients undergo placement of SBI they should be tested for immune markers of AID. In general, all women who want prostheses should have a thorough history and physical examination, emphasizing AID.
Our study has some limitations in that it has a retrospective design and with its different biases. We evaluated a select group of patients who were referred because they had some complaints of AID; however, there are many women who have undergone SBI placement who do not have clinical manifestations of autoimmunity or these are minimal.
This figure (*) shows multinucleated giant cell, surrounded by histiocytes. Courtesy of Socrates García Gutierrez MD. Department of Pathological Anatomy. Hospital de Especialidades, Centro Médico Nacional La Raza.
In patients with ASIA-SBI, family history of AID and environmental factors were associated with AID. Environmental and genetic factors for the development of AID must be taken into account in patients undergoing SBI in order to prevent the development of ASIA-SBI.
Citation: Vera-Lastra O, Cruz-DomÃÂÂnguez MP, Medrado RamÃÂÂrez G, Medina G, Amigo MC, Peralta-Amaro AL, et al. (2019) Autoimmune/Inflammatory Syndrome Induced by Silicone Breast Implant and Risk Factors Associated to Autoimmune Diseases. Rheumatology (Sunnyvale). 9:248. doi: 10.35248/2161-1149.19.9.248
Received: 17-May-2019 Accepted: 01-Jul-2019 Published: 08-Jul-2019 , DOI: 10.35248/2161-1149.19.9.248
Copyright: © 2019 Vera-Lastra O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.