Research Article - (2023)Volume 4, Issue 3
Hypothalamic Pituitary Gonadal Axis (HPG) operates and gated by multiple neuro-circuits one of which is circadian system. Circadian system is necessary for reproduction. The impact of day, night length, electromagnetic radiation, hormone and food on circadian cycle and onset of puberty was well established but influence of two crucial visual and auditory mating cues was not yet determined. Biological clock Suprachiasmatic Nucleus (SCN) is connected to other reproduction regulating areas such as Preoptic Area (POA) Antero-Ventral Periventricular Nucleus (AVPV) Arcuate Nucleus (ARC) and Dorsomedial Hypothalamus (DMH). Our finding has proved exposure of Visual and auditory mating cues significantly increased circadian clock regulatory protein expression GnRH neuropeptide and neurohormone that impart confounding impact on reproductive organ growth and early onset of puberty in prepubertal monkey (Macaca mulatto). The auditory and visual mating cues are thought to stimulate the SCN and Gonadotropin Releasing Hormone (GnRH) neuron in POA with subsequent released FSH, LH and gonadal steroids.
Circadian rhythms; Protein; Visual mating; Reproduction; Hormones
Puberty is marked by maturation of genital organs and development of secondary sex characters linked with time of becoming first able to sexually, reproducing [1]. Events that are initiated within the central nervous system recruit sexual development, puberty, adult fertility and need neural network maturation and function, that transfers both external and homeostatic cues to the separate hypothalamic neuronal group associated with secretion of Gonadotropin Releasing Hormone (GnRH) within the median eminence from neuroendocrine terminals into the pituitary hypophyseal portal vessel to regulate the secretion of gonadotropins (Luteinizing Hormone, LH and Follicle Stimulating Hormone, FSH) [2-7]. Under the influence of these gonadotropins ovaries and testes secrete steroids and initiate the production of sperm and eggs. Pubertal development associated with physical and behavioral vicissitudes, linked with activation of the hypothalamic adenohypophyseal–gonadal axis [8].
Gonadotropin-Releasing Hormone (GnRH) neurons are enmeshed intricately with the biological clock (SCN) and extensively studied for their contribution in the rhythmic release of gonadotropins and steroids hormones [9-11]. Kisspeptin neurons, the potent regulators of GnRH neurons are under investigation, possessing a similar circadian system accomplished a synchronized functional mechanism in the HPG axis. Inherently high numbers of kisspeptin neurons are not localized in in reproduction associated areas of the hypothalamus; they attain their adult numbers, developmental maturation and activity profile via developmental process [12-16]. Kisspeptin neuronal system is likely to “time” reproduction during development and aging by regulating circadian or estrus reproductive cycle as well as pulsatile GnRH/LH secretion. The promising role of day, night length, environmental influences, impact of various hormones on circadian cycle and associated GnRH neuronal neural network is well studied. Numerous brain circuits converge to sustain the rhythmic activation of the HPG axis, achieved by rhythmic cues originating mainly from the central Suprachiasmatic Nucleus (SCN). Anatomically the SCN divided into two nuclei, a core and a shell, termed the dorsomedial (dm) and ventrolateral (vl) SCN, characterized by the composition of neuropeptide. (vl)SCN mainly release Vasoactive Intestinal Peptide (VIP) and contain mostly neuropeptide releasing neurons comprised about 10% of total SCN, while AVP synthesizing neurons are confined in the (dm) SCN. The (vl)SCN function as the Rhythmical conductor where synchronizing cues were transmitted to the (dm)SCN, which in turn function as amplifier, transmit signal to slave oscillators located in other brain regions.
The POA area has denser innervation from VIP fibers, and a subpopulation of GnRH neurons (± 40%) within different nuclei of the HPG axis as they constitute cognate receptors, distinction in these factors contribute to early and late onset of puberty and sterility in some individual. Regulated release of gonadotropin hormones, Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH), marked the onset of puberty depend upon (GnRH) neural network, neuropeptide Gonadotropin releasing hormone, kisspeptin (kiss) circadian clock protein Period (Per), Cryptochrome (Cry), clock and bmal1 regulate effective and pulsatile release of GnRH. Rhythmic mRNA expression of clock genes, such as Bmal1, Per1 and Per2, revealed synchronization with oscillations in GnRH neurons GT1-7 cell line.
Our studies have revealed the striking influence of two potent visual and auditory mating signal on prepubertal rhesus monkeys with subsequent marked higher level gene expression of GnRH, cryptochrome, clock, bmal1, FSH and LH associated with morphological changes trigger early onset of puberty. The circulating level of GnRH, FSH and LH was increased in cues exposed monkeys administered at various developmental stages compared to control.
Interaction among the central biological clock, auditory, visual mating cues input and reproductive neuron in hypothalamicpituitary- gonadal axis of monkey induces precocious puberty. The Suprachiasmatic Nucleus (SCN), the central biological clock, can be divided into two major subdivisions known as the ventrolateral (vl) SCN, the core, and the dorsomedial (dm) SCN, the shell. The former contains cell bodies of Vasoactive Intestinal Polypeptide (VIP) neurons and the latter contains cell bodies of Arginine Vasopressin (AVP) neurons. The vl SCN acts as the conductor of rhythmicity and transmits synchronizing cues to the dm SCN. VIP neurons project to Gonadotropin Releasing Hormone (GnRH) neurons in the Preoptic Area (POA), whereas AVP neurons project to kisspeptin (Kiss) neurons in the Antero-Ventral Periventricular nucleus (AVPV). Auditory and visual mating cues are thought to stimulate the SCN and GnRH neuron in POA. The proposed mechanism was as illustrated in Figure 1.
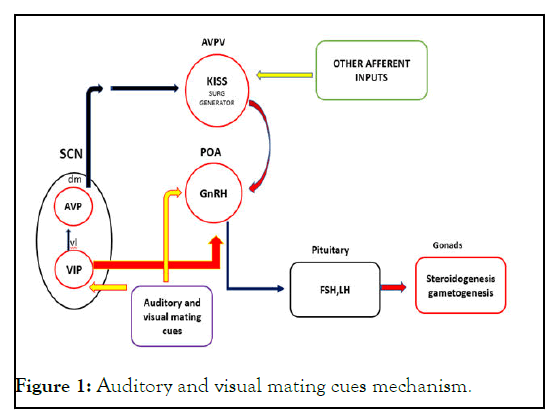
Figure 1: Auditory and visual mating cues mechanism.
Animal experiment
Twenty Prepubertal Rhesus monkey (age 100 week) were obtained and were divided into four groups. All the animals were provided food and water ad libitum and were kept in ventilated rooms at 25 ± 2 temperature with 12 h light-dark cycle.
Group1: Pre pubertal female and orchidectomized male monkey were taken as control. The control group was constituted of two animals, one pre pubertal female and orchidoectomized male=(1+1).
Group2: Female and orchidectomized male pre pubertal monkey were exposed to auditory mating behavior cues for 15 minutes. Cues were exposed for every twice in a week from age 100th to 160th week. Six animals were placed in this group N=(3+3).
Group3: Six female and orchidectomized male pre pubertal monkeys N=(3+3) were exposed to visual mating cues for 15 minutes with interval of twice in a week from age 100 week. to 160 week.
Group 4: Six female and orchidectomized male pre pubertal monkeys N=(3+3) were exposed to both auditory and visual mating cues for 15 minutes with interval of twice in a week from age 100 week. to 160 week.
Monkeys were placed in experimental chamber during the course of experiment with height of 5’,8” feet, width 2’.8” feet, and a window of optical glass through which the mating cues were exposed to monkey comprised of 1.8”,1.2” feet. The height of chair inside the chamber was 3.2 feet as depicted in Figure 2.
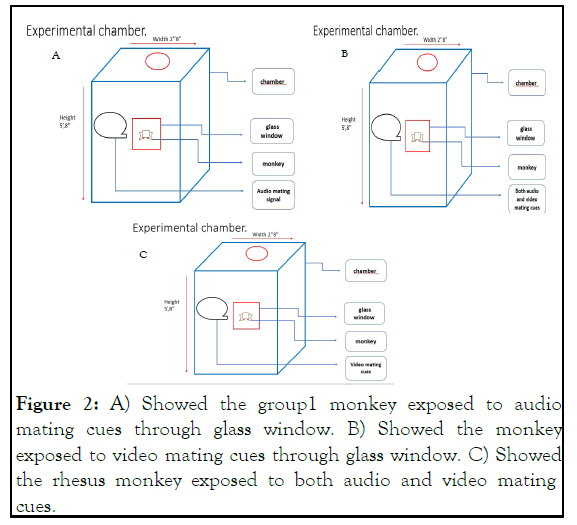
Figure 2: A) Showed the group1 monkey exposed to audio mating cues through glass window. B) Showed the monkey exposed to video mating cues through glass window. C) Showed the rhesus monkey exposed to both audio and video mating cues.
Catheterization
Monkeys were anaesthetized by (im) ketamix and two chronic indwelling cardiac catheters were implanted to permit withdrawal of sequential blood samples of monkeys via an internal jugular vein. Polyvinyl chloride and silastic tubings were used for the withdrawal blood sample. Animals were subsequently housed in remote sampling cages, which permitted continuous access to the venous circulation.
Blood sample collection
To describe changes in plasma gonadotropin concentrations, sequential blood samples (1.1 ml) were withdrawn via the sampling catheter as previously described and blood sample for GnRH plasma concentration was withdrawn from hypophyseal portal system by slaughtering animals.
Hormone assays
A detailed description of the radioimmunoassay procedures used for the determination of monkey LH, monkey FSH and GnRH has previously been published. The standard employed in both the FSH, LH and GnRH radioimmunoassay was a partially purified preparation of monkey pituitary gonadotropins (LER-M-907-D) which had an FSH potency of 0.26 NIH-FSH-Sl unit/mg (Steelman-Pohley assay) and an LH potency of 0.025 NIH-LH-Sl unit/mg (ovarian ascorbic acid depletion assay).
Hypothalamic neurons isolation by single molecule fluorescent in situ hybridization chain reaction on tissue section
The hypothalamic cells population was isolated by ‘single molecule Fluorescent In situ Hybridization technique’ sm (FISH). Hypothalamic tissues were flash frozen at 80 cryosectioned at 20 to create 10 μm sections from frost slides kept at 80. Slides were placed in 4% paraformaldehyde for 20 minutes at room temperature then washed with 70% ethanol 6 minutes before incubation in 70% ethanol for 2 hours. Slides were incubated in probe hybridization buffer in humidified chamber for 15 min at 37℃. Slides were incubated overnight in humidified chamber. After twenty hours the sections were washed with following solutions. (a) 70% probe wash buffer and 20% 5 × SSCT (SSC +10% Tween-20), (b) 45 % probe wash buffer and 45% 5 × SSCT, (c) 20% probe wash buffer and 80% 5 × SSCT and (d) 95% 5 × SSCT. Sides were washed at room temperature for 5 min in probe amplification buffer. Then 1 μl of hairpin were added in 100 μl of amplification buffer for preparation of hairpins. Then hairpin solution was frozen using thermocycler at 90℃ for 80 s then cooled at room temperature at the rate of 4℃/min. Amplification buffer was added in frozen hairpin solution to obtain desire volume. Slides were washed twice with 5 × SSCT at room temperature after overnight incubation in humidified chamber. Gold plus NucBlue fluoromount was added in desired amount. Slides were sealed with coverslips by clear nail polish and stored at 4℃ for imaging. List of probes used is as follows: BMAL1, CRY1, CLK, PER1, Kiss1, AVP, and VIP.
Circadian rhythm genes quantification by semiquantitative RT-PCR
Neuron cells were isolated from hypothalamus. Total cellular RNA was extracted from neuron cells using trizol reagent (Invitrogen) following manual. The one-step PCR kit (Qiagen, Chatsworth, CA) was used for semi-quantitative RT-PCR for the circadian rhythm genes, according to the manufacturer’s directions. PCR amplifications were performed with 1.25 U Taq polymerase (Life Technologies, Inc.) in a 50-l reaction 1 min at 94 C, 1 min at specific annealing temperature by using these primers.

And 1 min at 72℃. After determining the range of linearity of cDNA amplification, 100 ng RNA and 29 PCR cycles were used to amplify Per1, and cryptochrome1 (Cry1); 33 PCR cycles were used for BMAL1b and Kiss; 30 cycles were used for clock, AVP and VIP. The beta actin gene was amplified from 100 ng RNA for 30 cycles as a loading control. The ΔCt values of mating cues exposed cells were compared with control monkeys’ cells.
Body weight evaluations
The body weight of rhesus monkeys was measured by weigh balance at the end of experimental session and compared with control.
Statistical analysis
Statistical analysis was conducted using R statistical software package (version 3.3.1). And the graphs were generated with R.
Increased level of GnRH, FSH and LH concentration in audio, video and both mating cues exposed orchidectomized monkey from age 100 weeks-160 weeks
When male orchidectomized monkey of age 100-160 were exposed to auditory mating cues there were significant increase in serum concentration of GnRH, FSH and LH as compared to control. This increase in concentration was less than those concentrations measured in audio and video exposed monkey.
Concentration of GnRH: GnRH concentration in hypophysial portal circulation of orchidectomized monkey exposed to auditory mating cues achieved the normal concentration earlier than control monkey but not significant difference is determined. The same concentration in monkey exposed to video signal was achieved at age of 155 weeks compared to control.
Simillarly when monkeys were exposed to both auditory and visual mating cues same concentration is achieved at age of 152 weeks compared to 160 weeks as in control. At age 160 weeks in both cues exposed monkeys the concentration of GnRH is almost zero. These finding clearly indicate the potential role of mating cues and their differential exposure leads to early onset of GnRH surge and leads to early onset of puberty and sexual differentiation in monkeys as depicted in Figure 3.
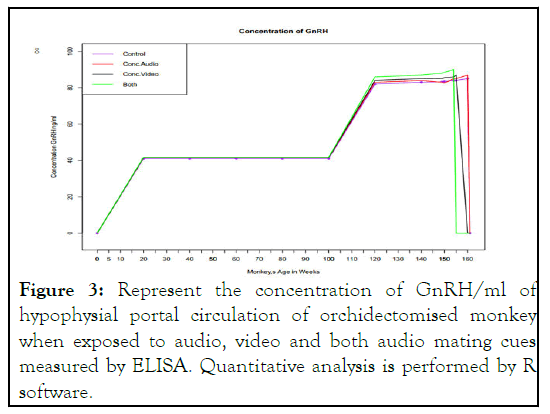
Figure 3: Represent the concentration of GnRH/ml of hypophysial portal circulation of orchidectomised monkey when exposed to audio, video and both audio mating cues measured by ELISA. Quantitative analysis is performed by R software.
Concentration of FSH: The concentration of FSH in orchidoectomized male monkey is 240 ng/ml at age of 160 weeks. After exposure to audio mating cues the same concentration of FSH is reached earlier at age of 155 weeks and concentration gradually decreases as age increases. Further we investigated the role of video signal on increasing the concentration of FSH. FSH concentration in response to video signal reached at peak value of 200 ng/ml concentration lower than normal value at age of 155 weeks and after this concentration gradually decreases as the age increases. This lower concentration may be due to different accuracy of ELISA reader. To investigate the effect of both audio and video mating cues we measure the serum concentration of FSH in orchidectomized pre pubertal monkey exposed to both cues interestingly results have shown that peak normal concentration of FSH is reached at age 152 weeks in exposed monkey indicate additive effect of both cues to release FSH. After that as age increases the concentration of FSH deceases. This effect is parallel to behavior of GnRH. So these results indicate potent role of these cues on development of sex organs and induce early spermatogenesis in male monkey as depicted in Figure 4.
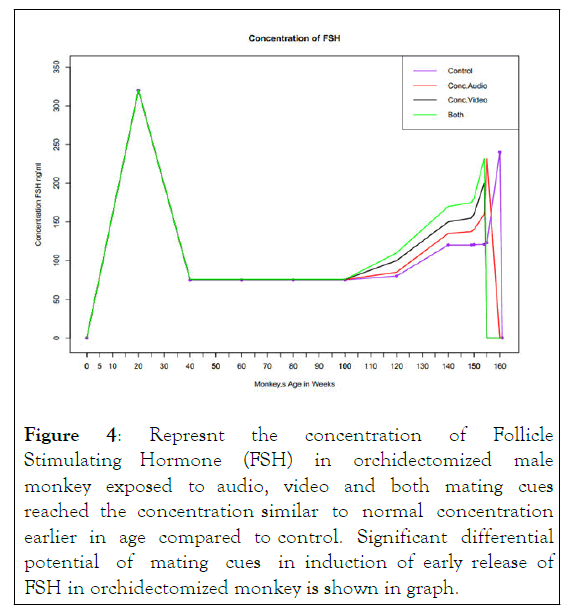
Figure 4: Represnt the concentration of Follicle Stimulating Hormone (FSH) in orchidectomized male monkey exposed to audio, video and both mating cues reached the concentration similar to normal concentration earlier in age compared to control. Significant differential potential of mating cues in induction of early release of FSH in orchidectomized monkey is shown in graph.
Concentration of LH: The concentration of LH in orchidectomized male monkey at the age of 150 weeks is 150 ng/ml. In audio mating signal exposed monkey, the same concentration of LH is reached earlier at age of 158 weeks, about a week after the peak concentration reached by FSH at age 157 week. After that concentration decreases as age increases. We find the effect of video mating cues on LH secretion from gonadotropes the peak concentration is reached at age 156th week. About one week after the peak concentration reached by FSH in video exposed monkey. These results indicate that video mating cues have more potent effect on release of GnRH, FSH and LH than audio cues. Further we find the effect of both audio and video mating cues on LH secretion. The peak concentration of LH was reached at 155th weeks in orchidectomized monkey and after this age concentration decreases indicate additive effect of both cues. Increase in weight and secondary sex character after exposure to cues is associated with increase in LH as depicted in Figure 5.
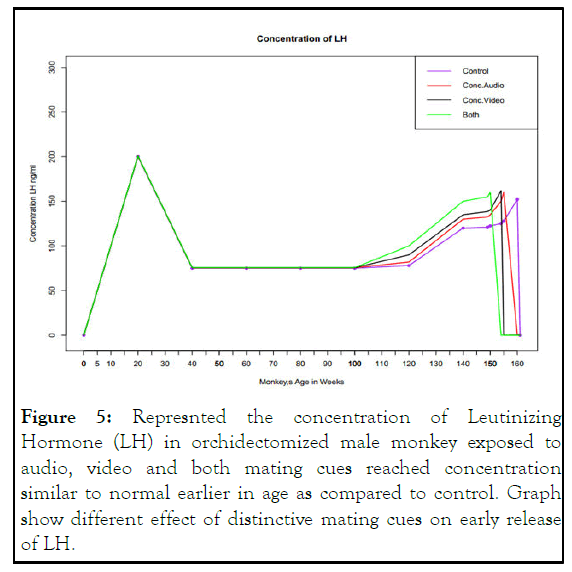
Figure 5: Represnted the concentration of Leutinizing Hormone (LH) in orchidectomized male monkey exposed to audio, video and both mating cues reached concentration similar to normal earlier in age as compared to control. Graph show different effect of distinctive mating cues on early release of LH.
The concentration of GnRH, FSH and LH was also increased in female monkey exposed to mating cues
To confirm whether the mating cues influence the reproductive hormone in intact male monkey we exposed firs monkey with audio mating cues second with video and third with both audio and video mating cues from age 100 week-150 week. Blood sample of each monkey was taken at last day of 150th week. Serum concentration of GnRH, FSH and LH was measured with ELISA. Results indicate the increase in concentration of GnRH, FSH, and LH in exposed monkey compared to control and both audio and video mating cues showed remarkable increase in concentration of hormone compared to control indicating additive effect of these cues as depicted in Figure 6.
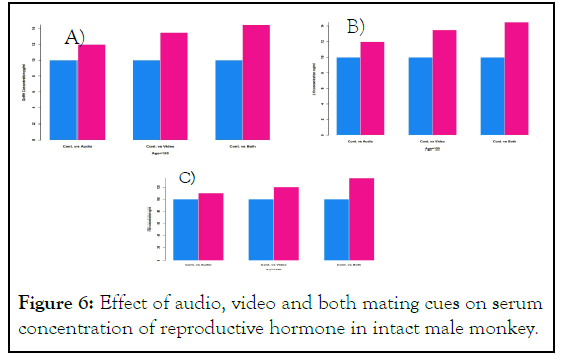
Figure 6: Effect of audio, video and both mating cues on serum concentration of reproductive hormone in intact male monkey.
These finding suggested that both audio and video mating cues play a remarkable effect on early onset of puberty and sexual maturation. The role of these cues on fertility and reproductive pathology is matter of concern.
Quantitative analysis is performed by R software. A) The serum concentration of GnRH in pg/ml significantly increased in mating cues exposed monkey blood at last day of 150th week compared to the control. Both audio and video showed additive effect on serum concentration of GnRH. B) The serum concentration of FSH in ng/ml significantly increased in mating cues exposed monkey blood at last day of 150th week compared to control. Both audio and video cues represent additive effect on FSH concentration parallel to GnRH. C) Showed the serum concentration of LH in ng/ml in mating cues exposed intact monkey at last day of age 150th week. It again describes the differential effect of mating cues on increment of serum LH concentration.
Effect of audio, video and both mating cues on GnRH neuronal network gene expression and protein concentration
In order to find how the concentration of GnRH was increased in mating cues exposed orchidectomized monkey we perform the protein analysis of GnRH neuronal network kisspeptin in Anteroventral Periventricular (AVPV) and Arcuate nucleus (ARC) and circadian clock regulating protein in Suprachiasmatic Nucleus (SCN) in shell neuron Arginine Vasopressin (AVP) and in core neuron Vasoactive Intestinal Peptide (VIP) the suprachiasmatic nucleus contain about 10000 neurons each is devoted for controlling the rhythmic or pulsatile release of GnRH . The underlying molecular autoregulatory feedback loop governs this pulsatile release. We investigated the effect of mating cues on protein controlling this feedback loop. Results have shown that beside the light, dark, the exogenous mating cues.
Increases the protein expression of Cryptochrome (CRY) period (PER1) brain and muscle ARNT like 1 (BMAL1) and CLOCK as compared to control. So these finding indicate that mating cues act at GnRH neuronal network predominately at SCN biological clock to elevate the GnRH concentration in circulation. However the mechanism by which the mating cues increase the expression of kisspetin is still elucidated. Its suggested that it was due to reciprocal activation of kisspeptin neuron in AVPV and ARC nucleus as these neuron express v1α receptor for AVP. The expression of v1α receptor on ARC neuron is not determined yet as depicted in Figure 7.
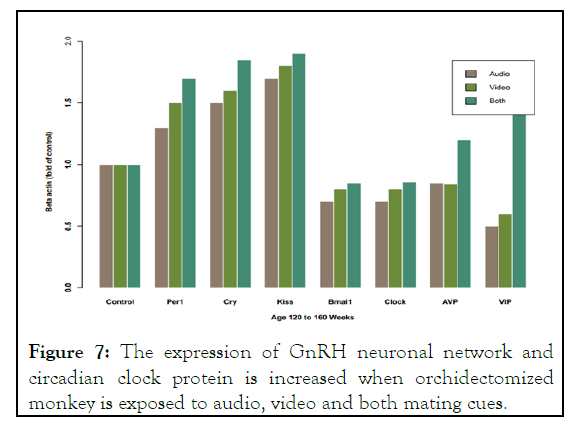
Figure 7: The expression of GnRH neuronal network and circadian clock protein is increased when orchidectomized monkey is exposed to audio, video and both mating cues.
Early development of physiological features “body weight” in mating cues exposed intact male monkey
The body weight of male monkey exposed to audio, video and both mating cues were shown to increases as compared to control at last day of age 150th week. The more pronounced effect is shown in monkey exposed to both audio and video mating signals. The body weight of control monkey at age 150th week was 7.5 kg. After exposure to audio signal the testes size significantly increase 35 g/testes with corresponding increase in body weight from 7.5 to 7.8. In monkey exposed to video mating signal the testes size rises from 35 g/testes to 45 g/testes and body weight increased from 7.8 to 8. Cumulative effect is shown on testes size and body weight of monkey exposed to both audio and video mating cues as testes weight increases from 25 g/testes to 50 g/testes and body weight increased from 7.5 kg to 8.2 kg at age of 150th week.
These results clearly indicate the effect of mating cues in histomorphological development of sex organs earlier than control monkey of similar age as depicted in Figure 8.
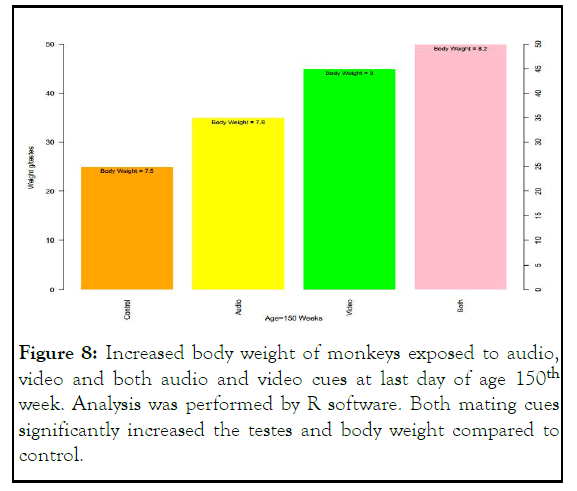
Figure 8: Increased body weight of monkeys exposed to audio, video and both audio and video cues at last day of age 150th week. Analysis was performed by R software. Both mating cues significantly increased the testes and body weight compared to control.
Our studies firstly reported the potential effect of audio, video and both mating cues on early onset of puberty in rhesus monkey. The finding demonstrated that puberty in primate can be advanced by intermittent exposure to visual, auditory and both mating cues as in presence of bucks, in the situation of a “male-effect” LH pulse frequency was elevated in adult does. The results reveled the potential impact of visual and auditory mating cues on LH frequency surge as puberty ensued. We evaluated the hormonal profile of orchidectomised male monkeys and normal female monkey from week 100 to 160. The LH surge was reached at peak level earlier when monkeys were exposed to both mating cues, similarly as in ewe lambs exposed to males, rams during the breeding season, with 0.33 pulses per hour in the control group vs. 0.59 pulses per hour in the group. After a male takeover, young females were expected to mature three times more. The fecal estrogen examination has raised sign in these species, that just prior to maturation of male a surge in estrogens increased in immature females of all age’s even females that did not mature as male takeovers. These are the first data to demonstrate that onset of maturation occur in close vicinity of specific males and were showed within a wild primate providing a conceivable mechanism for this change. Similarly, our results have shown increased in GnRH, FSH and LH concentration in orchidectomized monkey exposed to audio and video mating cues earlier than control mokeys revealed the mating cues associated earlier onset of puberty. Transcriptional autoregulatory loop maintained circadian rhythmicity of SCN neurons by the clock genes and their products. Photic signals transcriptionally activated, clock genes, such as period 1 (Per1) and Period 2 (Per2), maintain the expression pattern of brain and muscle ARNT like 1 (Bmal1), which dimerizes with (clock) “a circadian locomotor cycle kaput” to elevate transcriptional activity of circadian rhythm clock genes, such as Bmal1, Per1 and Per2, showed synchronicity of mRNA expression with oscillation of GnRH in GT1-7 Cell line. The GnRH neurons intrinsic rhythmicity suggests that components of the circadian oscillator may express in these cells. The expression of the circadian rhythm genes, clock, BMAL1, timeless (tim), period1, period2, cryptochrome1, and cryptochrome 2 was demonstrated in the GT1-7 cell line. Moreover, semi-quantitative RT-PCR revealed that GnRH mRNAs, BMAL1, as well as period1, and period2 are transcribed with a circadian-like rhythm after synchronization over 54 h. The pulsatile GnRH secretion was governed by intrinsic circadian molecular machinery of GnRH neurons as GnRH pulse frequency significantly decreased in mutated clock gene. Additionally, GnRH pulse amplitude increased by overexpression of cry gene, without altering pulse frequency. The sub-fertile attributes of GnRHspecific Bmal1 knockout mice, characterized by irregular secretion of LH while normal reproductive processes maintained including estrous cycle showed by in vivo study. These finding revealed that, to achieve regular GnRH/LH secretion, GnRH neurons need to be properly regulated by other neurons, though GnRH neurons have intrinsic molecular timing machinery. GnRH neurons received VIPergic afferents from the vl SCN and possess VPAC2 receptor that regulates circadian activity. The fiber networking between the SCN and reproductive nuclei such as the POA, AVPV, ARC, and DMH, proposed that crosstalk among the central biological clock and reproductive neurons ensure optimal reproductive function. The molecular mechanisms of light impact on GnRH neurons have intrinsic molecular timing machinery have been studied extensively. The neurotransmitter of the RHT glutamate induces phosphorylation of Ca2+-Camp Response Element Binding (CREB) protein as photic signals are directed to the SCN core consequentially in the transcription of some key clock genes. Our results revealed that in addition to other external cues responsible for central biological clock gene expression, audio and video mating cues potentially initiate the circadian rhythm genes, clock genes, such as Period 1 (Per1) and Period 2 (Per2), BMAL, VIP, CLOCK, Kisspeptin, cryptochrome (CRY). Expression of these protein activate GnRH neuron consequentially earlier pulsatile GnRH release initiate adrenarche, gonarche and thelarche and cause precautious puberty in rhesus monkeys (Macaca mulatto). There is some evidence from rhesus macaques in support of body weight influencing puberty onset. For example, macaque starting puberty at 3.5 year of age weighted significantly more at start of the breeding season than male reached puberty one year later at 4.5 years of age. In addition, body weight and testicular volumes were significantly correlated at 3.5 years of age. The ovaries weight of INTintact male exposed females tended to be higher relative to body weight in than other groups. Similarly, our study showed significant increase in body weight of intact monkey exposed to audio, video and both audio and video cues at last day of age 150th week. All of these finding demonstrated a potential effect of mating cues on onset of precocious puberty.
Although previous studies revealed the effect of social cues upon onset of puberty in macaques however, there were limitations as they did not elaborate the effect of mating cues on earlier onset of puberty and its mechanism.
Puberty presents the interaction of social and hormonal level of individual and is regulated by environment and social cues. During development there are various points that affect the onset and speed of puberty occurrence. The neuroendocrine mechanism controlling the onset of puberty appears to be sensitive to social and environmental cues. Various oscillatory mechanisms in neuronal network of SCN, POA, AVPV, ARC, regulate the pulsatile release of GnRH and timing of puberty. The timely activation of the HPG axis being maintained by many circuits in brain: This maintenance achieved by rhythmic cues received from Suprachiasmatic Nucleus (SCN). Our study revealed that central biological clock (SCN) and preoptic POA neuron receive visual and auditory mating cues input and activates Hypothalamic Pituitary Gonadal axis (HPG). This interaction among the central biological clock, auditory, visual mating cues input and reproductive neuron in hypothalamicpituitary- gonadal axis of monkeys initiate precocious puberty.
The study was conducted in KOC university graduate school of health sciences. All methods were performed in accordance with relevant guidelines and regulations of “local ethics committee for animal experiments of KOC university.” The animals were kept in the KOC University, Animal Research Facility (KUARF) of centre for translational medicine (KUTTAM). The study was approved by the committee with approval No. (2022-09).
The study is reported in accordance with arrive guidelines.
Not applicable.
The data used during the current study is demonstrated in this article.
I hereby declare that the work presented in the article is my own effort, and that the article is my own composition. A preprint has previously been published.
The author is thankful to KOC university animal research facilities, Istanbul Turkey for providing monkeys for experimental purpose.
No funding was available.
The author declared consent to publish.
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
Citation: Aslam M (2023) Auditory and Visual Mating Cues Input, Interaction among the Central Biological clock and Reproductive Neuron in Hypothalamic-Pituitary-Gonadal Axis of Rhesus Monkey: A Potential cause of Precocious Puberty. J Mol Pathol Biochem. 4:152.
Received: 16-Feb-2023, Manuscript No. JMPB-23-21820; Editor assigned: 20-Feb-2023, Pre QC No. JMPB-23-21820 (PQ); Reviewed: 06-Mar-2023, QC No. JMPB-23-21820; Revised: 05-May-2023, Manuscript No. JMPB-23-21820 (R); Published: 12-May-2023 , DOI: 10.35248/ JMPB.23.4.152
Copyright: © 2023 Aslam M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.