
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2022)Volume 12, Issue 2
Buprofezin (BPFN) is a thiadiazine insecticide that inhibits chitin synthesis and the moulting in case of white flies, mealybugs and leaf hoppers. The exposed insects are unable to shed their cuticle and ultimately die as moulting ensue. Neurobehavioral toxic effects elicited by buprofezin remained unclear. Furthermore, the reversal of buprofezin induced neurobehavioral toxicity by atropine was not elaborated. Thus, we explored the neurobehavioral toxic consequences of acute buprofezin exposure in adult male rats and effective reversal of these changes by pretreatment with atropine as an antidote. Acute administration of commercial buprofezin (87.9 mg/kg/day through oral gavage with corn oil as vehicle) induce a wide range of neurobehavioral toxicity including damage to pyramidal cells of hippocampal Carnu Ammonis (CA1), and CA3, region and behavioral impairments as demonstrated through, loss of motor coordination, locomotor activity, fear loss, hearing, heat shock, sensorimotor, cognitive and spatial navigation impairment following the exposure. These neurobehavioral toxic effect of acute buprofezin exposure were significantly reversed by the 15 min pre- treatment of atropine antidote before the buprofezin administration. Pre-treated atropine (20 mg/kg/day; IP) attenuates the neurobehavioral toxicity induced by buprofezin in adult male rats. It was suggested that acute buprofezin exposure elevated the Acetylcholine level, by inhibiting the synthesis and release of Acetylcholine Esterase (AChE) in synapse. But the complete mechanisms are remained to be elucidated.
Buprofezin; Atropine; Thiadiazine; Acetylcholinesterase; Moulting; CA1; CA3
Pesticides are extensively used in commercial and agriculture fields to control growth of pest [1]. The abundance of toxic pesticides has been banned in most countries, because they are classified as organic pollutant [2], yet their component and metabolic residues are still found in environment and human [3]. Several pesticides are constructed in order to attack on nervous system of pests, so due to the similar neurochemical transmission system these insecticides are neurotoxic to human at different doses.
The ubiquitous application of pesticides in distinct areas has elevated the risk of environmental pollution by various xenobiotics that can be highly toxic for non-target organism containing human. Potential hazards of these pesticides, involving their neurotoxic effects on development have been reported in previous finding [4].
The buprofezin ((Z)-2-tert-butylimino-3-isopropyle-5-phenyle-1,3,5- thiadiazin-4-one) belongs to thiadiazine class of pesticides that inhibit the molting process of different pests including white flies, mealybugs and leaf hoppers. [5]. In insects it inhibits the chitin synthesis and showed its action as the molting process proceeded.
The exposed insects are unable to shed their cuticle and ultimately die during this molting process [6]. BPFN render incorporation of 3H-glucosamin into chitin [7]. Because of chitin deficiency, the elasticity of procuticle is lost in whitefly nymphs and the insect was incapable to accomplish the molting [8]. Soil residing buprofezin was decayed into various metabolites by soil living microorganisms [9]. Delineate transformation pathway of buprofezin via Pseudomonas sp DFS35-4 a strain that metabolize buprofezin present in polluted China. Rice field soil residing Rhodococcus sp Strain YL-1, has capability of biodegrading buprofezin into four metabolites: 2-isothiocyanato-2-methyl-propane, 2-tert-butylimino- 3-isopropyl-1,3,5-thiadiazinan-4-one, N-tert-butyl-thioformimidic acid formylaminomethylester, and 2-isothiocyanato-propane [10].
The buprofezin acute toxicity was non-significant in earthworm and fishes, mammals and eating birds, but was highly toxic to aquatic ecosystem. BPFN get accumulated in aquatic milieu thus buprofezin exposed aquatic ecosystem is at hazard. Buprofezin chronic toxicity for aquatic organism is absent, though it is extensively used for regulation of insect growth. Acute exposure to buprofezin (dermal, oral or through respiration) induce low toxicity in mammals oral and dermal LD50>2000 mg/kg body weight. LC50 4.57 mg/L air/4th and do not produce itching in skin and eyes [11].
Administration of 5000 ppm buprofezin to sprague dawley rats enlarges liver and thyroid and turn them dark brownish color [12]. Alteration in serum concentration of SGPT, SGOT, urea and creatinine, thiobarbituric acid, Reactive Oxygen Species tissues of liver and kidney. Associated substantial reduction of total protein, antioxidants enzymes, the SOD, CAT, POD, and GSH non-enzymatic reduced glutathione. Damage was showed in hitomorphology of liver and kidney. Damage of liver hepatocytes, shrinkage of kidney glomerulus rupture capillaries, necrosis of tubular epithelial cell and increased Bowman’s space were observed after acute exposure of buprofezin in Balb/c mice [13].
Liver and thyroid are adversely affected by buprofezin and histopathological changes not occur in affected organism but it changes clinical chemistry. Oral administration of buprofezin for two days induce micronuclei in bone marrow erythrocyte of mouse. BPFN devoid carcinogenicity and reproductive toxicity in rats or mice and not induce neurotoxicity in mammals [14]. Buprofezin acts on liver the main site of its action. Various researches suggested that BPFN accumulated in liver and consequently induces oxidative stress. Cytochrome C oxidase activity is retarded by buprofezin, which is most important cause of energy production and thus produce (ROS) [15]. Buprofezin induced chronic toxicity and carcinogenicity has suggested that liver is chief toxicity target in mice and rats. A two-year study in mice revealed that body weight of female and male mice decreased from week 6 (male) and week 9 (female) onward at 5000 ppm dose, but a little decreased at 2000 ppm dose [12].
In earthworm the activity of AChE was intensely repressed by lufenuron subsequently by buprofezin, and then triflumuron in descendant [16]. The study on B. tabaci found all over the word horticulture, agriculture and ornamental plants have revealed that buprofezin is being used as AChE blocker to kill insects that had developed resistant to organophosphate and carbamates [17]. It is clearly established that (CNS) is essential for production of behavior. It is also clearly recognized that human and animal behavior is altered by a several of chemical’s entities. The behavior measure has been recognized as essential in screening the potential toxicity of these agents on central nervous system. This recognition has opened a new area of research within toxicology which has been referred by Weiss and Laties (1969) as” behavioral Toxicology” and by Zbinden (1983) as “neurobehavioral toxicology. With the advancing incidents of neurobehavioral and cognitive defects the objective of this study was to investigate the neurobehavioral toxicity, underlying mechanism, and possible therapeutic approach to overcome its neurotoxicity following acute exposure of buprofezin [18].
Animals
For this study twenty-eight sprague dawley adult male rats (16-17 weeks old) weighing 200-240 gm were obtained from primate facility department of animal sciences Quaid-I-Azam university Islamabad and were accommodated in same department. Rats were placed in steel cages constituting seven rats in each cage in a standard temperature of 25°C and 10/14 h light and dark period, with free availability of rodent diet and tap water. The experiment was conducted according the guidelines of “Bioethical Committee of Biological Sciences” on use and care of animals and was approved by the bioethical committee of university.
Experimental design
The rats were separated randomly and divided into groups. First group was taken as control second group was treated with buprofezin 87.9 mg/kg/day through oral gavage with corn oil for seven days. Third group was pre-treated with atropine 20 mg/kg/ day given Intraperiotoneal (IP) and after fifteen minutes followed by administration of buprofezin 87.9 mg/kg/day for seven days. Seven rats were placed in additional group for assessment of death latency. Author suggested that BPFN reduced the synaptic level of Acetylcholinesterase (AChE) in synapse subsequently elevated Acetylcholine (Ach) results excitotoxicity and neuronal death occur. Logically pretreated competitive antagonist atropine mitigated the elevated Ach mediated neuronal death. All rats underwent behavioral tests before sacrificing.
Chemical
Commercial formulation of buprofezin (Robon 250 g/kg 25% w/w) linear formula C16H23N2OS and molecular weight 305.44 g/mol CAS NO. 69327-76-0 was purchased from Jaffer group of companies. Buprofezin is a 2-(tert-butylimino)-5-phenyl-3-(propan- 2-yl)-1, 3, 5-thiadiazinan-4-one in which the C=N double bond contain Z conformation. It is a member of homopteran inhibitor of chitin biosynthesis works as an insecticide. Atropine (20 mg/kg) and neostigmine were purchased from Sigma-Aldrich (SP, Brazil).
Histological analysis of brain
At the 7th day of treatment rats were anesthetized by (IP) administration of ketamix (0.1 ml/kg body weight) and sacrificed to obtained body organs. The Brains were dissected out, weighed and subsequently immersed in freshly prepared 4% paraformaldehyde for 16 hours at 4°C and processed for paraffin embedding. Tissue blocks containing hippocampus (-3.4 mm to -3.8 mm posterior to bregma) were further processed for paraffin embedding and 8 μ thick serial section were cut in coronal plane under microtome. The sections were stained with hematoxylin-eosin (H and E) conventional method and mounted. Hematoxylin-eosin was performed to investigate the structural changes following the buprofezin exposure and its protection with pre-treated atropine.
Motor coordination
Motor coordination was evaluated by using rotarod test, which is performed by placing rats on rotating rod that rotates around its axis; horizontal and parallel bars. Rats were placed 25-30 min in testing room prior to testing.
Rotarod test
A rod of adjustable diameter (10.64 cm) was rotated at about 4 rpm. The rotation velocity was gradually increased until the rat fell off the rotating rod. When the increasing speed is measured, the time from start until the rat’s fall off consider as measure of motor ability [19-22]. The motor coordination of control, buprofezin treated and pre-treated atropine plus buprofezin rat was quantitatively evaluated using accelerating rota-rod for rats with laser rpm calculator. The rotarod was 10.64 cm in diameter constituted from steel rod with knurled surface for treading. Two circular plastic glass disks were fixed at the ends of the rod with diameter of 40.64 cm. The disks prohibit escape and worked as a barrier between rats. The rotarod was affixed 71.12 cm above the floor. Rats were mounted on the rotarod perpendicular to the long axis of the rod, with their heads facing away from the observer. As rat was mounted on the rod, the run experiment menu allows the experimenter runs experiments. When the experiment is running the current speed of rotarod is displayed on LASER rpm calculator, as well as the amount of elapsed time as the experiment has started was measured with stop watch. As the rat falls from the rotarod, the rotational velocity of the rotarod at the time of the fall is showed by LASER rpm along with the amount of time the rat elapsed on rod was calculated manually by stop watch.
Horizontal bars test
The "string test" 1, "coat hanger test" or horizontal bar test was used to measure forelimb strength and coordination. We have noticed that performance depends on tightness of string therefore most experiments use a metal bar. It is noted that rat’s capability to grasp the bar is inversely proportionate to bar diameter and standardly used bar has 2 mm in diameter. The bar length was 91.4 cm and were lifted 73.6 cm above the floor by wooden supporting columns. We held the rats by the tail, gently put it on the table in front of the apparatus, rapidly drags it backwards almost 20 cm so that rats align perpendicular to the bar, swiftly pick up and allowed it to grip the horizontal bar at its middle only with its forepaws, and fully relax the tail, consecutively starting the stop watch. The criterion was to measure either a time of fall from the bar prior the rat reached one end of wooden column, or measurement of time until its one forepaw touches a supporting pillar. The optimum test time was 30 sec. If rat failed to grip bar before first 5 secs this was attributed to poor placing and this fall was not counted. If rat fall between 1-5 sec score=1, If rat fall 6-10 second score=2, If rat fall 11-20 second score=3, If rat fall 21-30 second score=4 and If rat fall >30 second score=5
Parallel bars test
Two parallel steel bars 91.4 cm in length and 4 mm diameter were fixed 2.5 cm apart by wooden supporting columns at their ends elevated 73.6 cm above the floor. Two parameters were measured. Maximum test time was 120 sec. Rats were held by the tail, gently put it on the bench in front of the apparatus, rapidly drags it backwards about 20 cm so that rats align perpendicular to the bar, swiftly pick up and allowed it to grip the horizontal bar at its middle only with its forepaws, and fully relax the tail, subsequently starting the stop watch. First criteria point was to measure time the rat takes to orient 90° from start. Second criteria point is to count the time taken by rat to reach at end of bar.
Fear conditioning
Fear conditioning tests were performed in fear conditioning test box comprised of light and dark compartments. Dark compartment was containing electric bell for cued and steel wire floor connected with electric supply for contextual fear conditioning. Length of each chamber was 73 cm each compartment was 36.8 cm long and wide. Both chambers contained window of 10 cm in diameter. Before experiment rats were permitted to acclimatize in testing room for 30 min.
For contextual fear conditioning test rats were placed in testing chamber and electric foot shock of 0.14 mA was imposed for 2 sec. Rats suddenly steps from shock compartment to other and freezing was examined for 5 min. For cued fear conditioning test rats were placed in same testing compartment and electric bell was turned on and electric current in steel wire was turned off, which delivered white noise tone of 90 dB for 30 sec. Rats suddenly steps in from bell containing chamber into other compartment and freezing was examined for 5 min.
For both cued and contextual fear conditioning rats were retained in conditioning test box and allowed to acclimatize for 2 min. Every animal then received a white noise tone of 90 dB for 30 seconds and after that an electric foot shock of 0.14 mA for 2 secs was subsequently delivered. The time duration between tone and shock was 2 min. After the exposure of last shock rats were left in chamber for 20 secs before removing. Freezing behavior was calculated for 5 min. Shock threshold for flinching, jumping and vocalization was measured of control, buprofezin and pretreated atropine plus buprofezin in passive avoidance test box provided with variable current in mA.
Step through passive avoidance
Rats were placed into passive avoidance test box containing a dark and light chamber. On the first day rats were placed into lighted chamber, and latency for the rat to step into dark chamber was recorded. Subsequently 0.2 mA shock of electricity was provided for 2 sec and rat was removed. On second day rat were placed into lighted compartment, and latency for rat to step into the dark chamber was measured again with maximum time of 5 min.
Time space navigation, rearing, total locomotion activity, working memory, reference memory and memory errors measurements
Time space navigation, rearing, total locomotion activity, working memory and reference memory was measured by using “TSD MAZE, CSS OR CLOSE MAZE”. The activity of Head Direction cells (HD) hippocampal Grid cells, place cells, speed cells and TSD cells was assessed by preference of path selection by rats when food stimulus was baited in the both ends of path. Rearing behavior consist of rats standing upright position on its both hind paws and is index of anxiety in TSD OR CSS MAZE and the elevated plus maze. Number of rearing was assessed in TSD maze for 15 minutes. The total locomotion activity of control, buprofezin treated and pre-treated atropine plus buprofezin was analyzed manually after release of rats in 705 cm long TSD maze. Rats were freely allowed to move in both long and short pathways. The total activity was measured in time scale of 15 min.
Evaluation of working and reference memory and memory errors in TSD or CSS Maze
Working memory can be demarcated as a memory for an object, recognition, or location that is used within a testing period, but not usually between the periods. It is variant from reference memory which is demarcated a memory that would typically be attained with rehearsal training and would sustain from days to months. The reference memory is mostly the memory for the ‘rules’ of a given chore. For instance, when testing object press a bar receive a food object or a water maze established with a hidden platform or entrances into the food containing pathway of the TSD or CSS Maze. Moreover, working memory enable the testing object to remember which pathway it had visited in a testing period.
Testing animal adaptation period
The rats were presented two periods of adaptation on two succeeding days before the learning process commences. The testing rats were allowed to walk around the food baited pathway of the maze for 15 min during the testing time. The testing rats were explored the TSD Maze baited with food stimulus first in long pathway, then food baited in short pathway and at the end food baited at both pathways and path acquisition in each case was recorded. Following the adaptation period, the acquisition process was ongoing.
Testing animal acquisition career
During the testing animal acquisition career or (learning session), the rats were assumed three trials of acquisition per day until the rats achieved the learning criteria. The learning criteria were confronted as follows. The trial was sustained for 15 min and the training was continuous until the rats achieved the criteria of 80% correct choice; i.e., at least four correct entries out of five. The duration of this session varied depending upon condition of research procedure the maze was washed with ethanol (70%) at start of trial session and thereafter one path was baited with food stimulus. For first trial the rat was kept in central box and was permitted to choose any pathway. When the rat reached the end of pathway and ate the bait reward, the path choice was noted. Only the first approach to the baited pathway was documented as a correct choice and the maze pathway. For second trial the pathway was rebaited and entries of rat in baited pathway were recorded. Entrances into the path containing no food stimulus were recorded as Reference Memory Errors (RME). For third trial the both pathways were baited with food stimulus and path entries of rat was recorded reentries into baited pathway when both pathways are baited referred as Working Memory Error (WME). For fourth trial the both pathways were baited and choice of short pathway was recorded as correctness of TSN. Each rat was assumed four trials per day and obtained data from the four trials were averaged and included in analysis of final data. The performance pattern of rats was recorded by the percentage of the correct choices, RME and TSN (Time Space Navigation) in TSD Maze, COSE Maze or CSS Maze.
Morris water maze test
A circular steel pool of diameter 125 cm blue color was used for testing. The pool was divided into four quadrants each with circumference of 208 cm. The height of pool was 60 cm and water level in visible plate form was 10 cm. We place the platform of 12 cm in diameter and 40.6 cm in height in pool. The pool was filled with water until the water level was 1 cm below the platform and allowed the water to equilibrate at room temperature 22°C. Hot water was added to maintain temperature. Rats were removed from their cages to behavior room. Rats were kept in area where they were not allowed to see the pool or spatial cues. Before testing they were acclimatized for at least 30 min. A flag was fixed on platform to increase visibility. Rats were gently lifted by tail and softly putted into water opposite to edge of pool. Allowed the rats to remain on the platform for 5-6 sec if the rat finds the platform before 60 sec then transfer it to home cage. Same procedure was followed for each trial. For visible platform (nonspatial) test. Rats were given four trials. For each trial the order of start position was changed. The platform search latency for each rat was recorded with stopwatch. The maximum time for rat to find platform was 60 sec. For hidden platform (spatial) water level was raised 1 cm above the platform and rats were trained to locate hidden platform. It is used for long term memory and acquiring spatial location of platform. Rats were given four trials and latency for rat to locate platform was measured in duration of 60 sec. For probe trial we create one trial and platform was removed. There is one starting direction farthest from platform quadrant. Platform search time and no of platform crossing was recorded for 60 sec. The rat starting direction and platform location in Morris water Maze is as follows.
Hind limb landing foot splay test
Landing foot splay was evaluated according to the procedure described by Bowen, et al. [23]. The tarsal joint of each hind foot of each rat was marked with a drop of colored ink and animal was held in a supine position and then dropped from a height of approximately 30 cm on to a white absorbent paper. The distance (cm) between two-foot prints of hind limbs was measured. This procedure was repeated three times for each animal and finally three readings were averaged.
Body weight
The average body weights of individual male rat of control group and treated were recorded at 7th day of experiments.
Hot plate tests
For hot plate rats were placed on heated plate at 60°C. Rapidly turn on stopwatch and latency for hind paw withdrawal was calculated. Licking, shaking and jumping of rat’s hind paw was observed. Latency by default was considered when rat did not show any reaction to heat after 30 sec.
Statistical analysis
The data was statistically analyzed with ONE WAY ANOVA followed by Tukey’s post hoc multiple comparison test with GraphPad Prism Software, version 5, lnc., La Jolla, CA, USA). Data was demonstrated as Mean ± SEM. P<0.05 represented the significant difference.
Brain-body weight index evaluations
Body weight of buprofezin exposed group was comparable to concurrent control and pre-treated atropine group at 7th day of treatment. The decrease in body weight was associated with loss of appetite and decreased food intake as a consequent of BPFN neuron damage. Pre-exposed atropine slightly attenuates the weight loss induced by buprofezin, (Figure 1A). The brain weight of acute BPFN treated rats have been found to be decreased, due to concomitant neuronal atrophy (Figure1B). The strong correlation was found between brain and body weight loss in treated rats. The brain-body weight index was measured for control and treated groups (Tables 1 and 2). The finding demonstrated substantial variation in brain-body weight index between groups and has been significantly affected by acute buprofezin exposure. Moreover, the aberrant value of brain-body weight index in pretreated atropine was consequent of its failure to significantly regain body weight following buprofezin exposure. Hence pretreated atropine revealed a paramount mitigated effect in brain weight, body weight and their coefficient.
| Day 1 | Day 2 probe test | ||
|---|---|---|---|
| Platform location | Starting direction | NO platform. Start direction | |
| Trial 1 | SW | S | N |
| Trial 2 | NE | N | |
| Trial 3 | NW | E | |
| Trial 4 | SE | W | |
| Trial 5 | Center | E | |
Table 1: The rat starting direction and platform location in Morris water maze is as follows.
| Parameters | Control (group) | Buprofezin (group) | Atropine+buprofezin (group) |
|---|---|---|---|
| Brain weight (Mean ± SEM) | 1.702 ± 0.035 | 1.594 ± 0.024 a* | 1.684 ± 0.030 b* |
| Body weight (Mean ± SEM) | 229 ± 0.57 | 209 ± 0.45 a* | 210 ± 0.52 a* |
| Brain-body weight index | 0.0074 | 0.0076 | 0.0088 |
Note: “a*” significant different from control. “b*” significant different from pre-treated atropine.
Table 2: Brain-body weight index of sprague dowley rat after acute exposure of buprofezin and pretreated atropine+buprofezin.
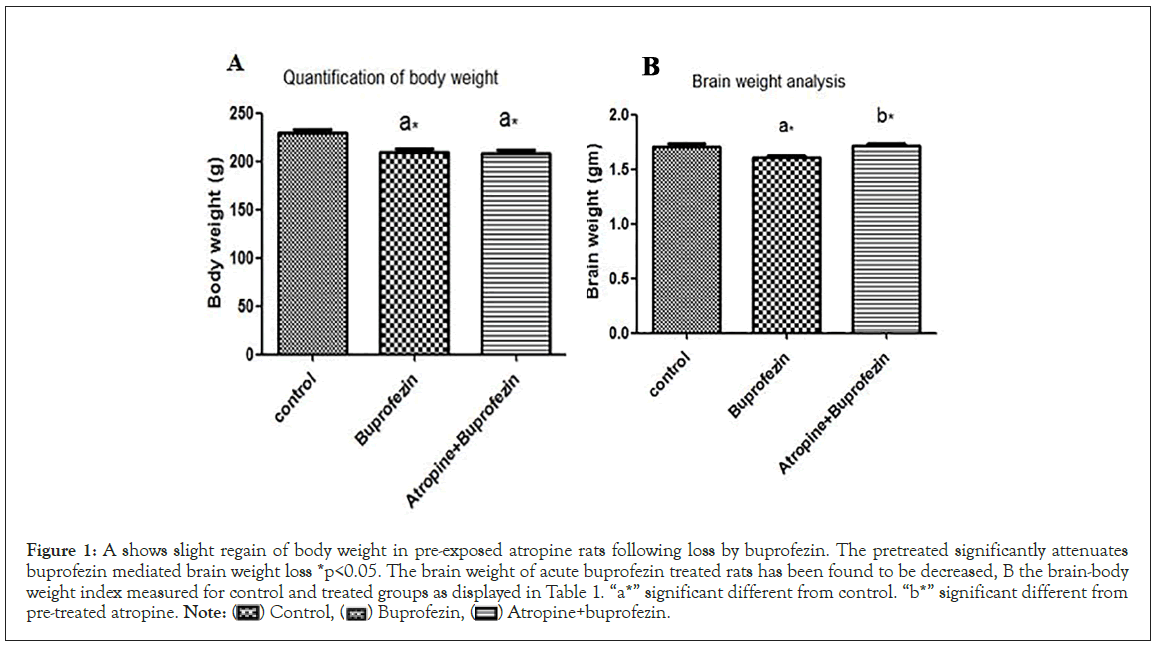
Figure 1: A shows slight regain of body weight in pre-exposed atropine rats following loss by buprofezin. The pretreated significantly attenuates
buprofezin mediated brain weight loss *p<0.05. The brain weight of acute buprofezin treated rats has been found to be decreased, B the brain-body
weight index measured for control and treated groups as displayed in Table 1. “a*” significant different from control. “b*” significant different from
pre-treated atropine. 
Buprofezin induced histopathological alterations of hippocampus were attenuated by pre-treated atropine
Morris water maze test and time space determinant maze finding demonstrated that acute buprofezin exposure impair spatial, working and reference memory as well as time space navigation. We concentrated on histopathological alteration of hippocampus provoked by buprofezin treatment. Hippocampus can be classified into several regions on the basis of pyramidal neuron morphology as CA1-CA4. We observe the changes in hippocampus with staining. Results demonstrate that buprofezin exposure induce alteration in various regions of hippocampus. It is reported that the function of pyramidal cells in CA1 region were attributed with long term memory and degeneration of CA1 pyramidal cells may cause significant memory loss. Thus, to analyze histopathological alteration and ultrastructure variation in response to buprofezin exposure CA1 and CA3 regions of hippocampus was analyzed by hematoxylin and eosin staining.
H and E staining showed that in control group pyramidal cells were arranged orderly with intact nuclei stained clear, dark blue. However, in acute buprofezin treated rat’s hippocampus H and E staining revealed more degenerative (apoptotic) neuron and pyramidal cells showed granular vacular changes and nuclear pyknosis (Figure 2). The cell junction and basement membrane were degenerated. In pre-treated atropine group pyramidal cell show better cell morphology compared to buprofezin treated. Cell’s junctions were intact and cells were in compact form. Only few cells underwent degenerative changes. These finding clearly demonstrate the ameliorative effect of pre-treated atropine against buprofezin neurotoxicity.
Figure 2: A shows that photomicrograph of 8 μm thick coronal section of rat hippocampus (-3.8 mm behind bregma) stained with Hematoxylin-
Eosin (HE). The panel of each column showed control, buprofezin treated and pre-treatred atropine plus buprofezin of CA1 and CA3 region of
hippocampus. (A) showed the apoptotic pyramidal cells. (P) showed the pyknotic cells. Normal cells are darkly stained. Disrupted and misalignment
of layers is shown in experimental group. However pre-treated atropine group showed less pyramidal neuron degeneration compared to buprofezin
treated group. B pretreated atropine attenuated the apoptosis and pyknosis of hippocampal pyramidal neurons in CA1 and CA3 regions. Acute
buprofezin exposure induce almost 60% cell loss in CA1, (40% apoptotic and 20% pyknotic) contrast to CA3, (25% apoptosis and 35% pyknotic)
and pretreated atropine has reduced cell to 34%. P value *p<0.05, **p<0.01, ***P<0.001. “a” significant different from control. “b” significant
different from apoptotic. 
Atropines reverse the impaired motor coordination induced by acute buprofezin exposure
Motor coordination of rats was analyzed by using rotarod. the results indicated that latency of fall from rotating rotarod was significantly reduced (P<0.001) in acute buprofezin exposed groups compared to control and pre-treated atropine counteract the toxic effect of buprofezin as there is less significant difference compared to control, (Figure 3A).
Figure 3: Showed the pre-treated atropine attenuated the buprofezin induced impairments in motor coordination. A) latency on rotarod was
significantly decreased by acute buprofezin administration and substantial regain occur with pre-treated atropine. P value *p<0.05, ***P<0.001.
B) limb grip strength was significantly reduced in buprofezin treated group and sufficiently reversed with pre-treated atropine. p value **p<0.01,
***P<0.001. C) buprofezin treated rat’s takes more time of orientation compared to control and pre-treated atropine showed mild effect. P value
*p<0.05, **P<0.01. D) exhibit the buprofezin treated rats takes more traverse time to reach support atropine showed a little effect. p value *p<0.05,
**P<0.01. E) pre-treated atropine significantly counteract the buprofezin induced decreased distance travelled on parallel bar in 120 sec. “a” significant
different from control. “b” significant different from pre-treated atropine. 
The forelimb grip strength and coordination were assessed by using horizontal and parallel bars. Results have shown statistically significant (P<0.001) impairment in fore limb grip strength in horizontal bars and was no significantly counteracted by atropine, (Figures 3B-3E).
In case of parallel bar time taken by rat to orient 90° and traverse time was significantly increased in buprofezin treated rats compared to control. Total distance on bars in given time duration was reduced in buprofezin exposed group. Pre-treated atropine has reversed the buprofezin effect as the there is no significant difference between control and pre-treated atropine plus buprofezin groups.
Buprofezin induced long term deficit of contextual and cued memory was revered by atropine
In behavior testing group of rats were trained by pairing of two sets of stimuli, mild foot shocks with an associated context and an auditory cue. After training session, we found that buprofezin treated rats’ exhibit significantly less freezing compared to control and pre-treated atropine plus buprofezin exhibited no significant change in freezing compared to control. These results suggested that pre-treated atropine counteracted the loss of fear conditioning caused by acute exposure of buprofezin. Initially we tested the cued then contextual and the lastly, we paired cued and context to examine the fear conditioning, (Figures 4A-4C).
Figure 4: A) demonstrate the loss of fear condition by acute exposure of buprofezin is significantly reversal by pre-treated atropine. P value ***P<0.001.
B) substantial reversal of cued fear against buprofezin induced loss of fear condition. P value **P<0.01; C) buprofezin induced loss of cued plus
contextual fear was significantly reverted by pre-treated atropine. P-value ***P<0.001. “a” significant different from control. “b” significant different
from pre-treated atropine. 
Atropine slightly counteract the impaired passive avoidance induced by acute exposure of buprofezin
In passive avoidance test the rat learned to decrease their natural tendency to avoid from light compartment to darker compartment of training chamber. We trained the rats by placing them into a lighted compartment and measured the latency after which the rat entered into dark chamber where they receive a mild electric foot shock. Next day we assessed the latency of rat to step into dark compartment. Our results suggested that first day rat spend significant greater time in lighted compartment compared to control. While on second day in spite of receiving foot shock buprofezin treated rat latency in lighted compartment was significantly reduced compared to control and was remarkably reversed by pre-atropine administration. These finding indicate the loss of sensory receptor by acute buprofezin exposure, (Figure 5).
Figure 5: plot showed the protective effect of pre-treated atropine against buprofezin induced loss of passive avoidance. Buprofezin treated
rat represent impaired passive avoidance after electric shock at day 2 and was slightly counteracted by pre- treated atropine. P value ***P<0.001,
**P<0.01.6. B) plot exhibit the quantification of shock threshold for flinching, jumping and vocalization in buprofezin treated and pre-treated
atropine rats. Pre-treated atropine significantly counteracts the increased in shock threshold for flinching, jumping and vocalization. P value *P<0.05,
**P<0.01. “a” significant different from control. “b” significant different from pre-treated atropine. 
Quantification of shock threshold for flinching, jumping and vocalization
Our results showed the remarkable increase in shock threshold for flinching, jumping and vocalization in buprofezin treated rats compared to control indicating loss of general sensory perception. Pre-treated atropine rats showed no significant increase in shock threshold compared to control, (Figure 6).
Figure 6: A) demonstrated the significant protective effect of pre-treated atropine against buprofezin induced anxiety (less no of rearing) in TSD
maze for 15 minutes’ duration. P value *P<0.05, **P<0.01***P<0.001. B) represent the substantial reversal of total locomotion activity by pre-treated
atropine caused by acute buprofezin exposure in 705 cm long TSD maze. *P<0.05, ***p<0.001. “a” significant different from control. “b” significant
different from pre-treated atropine. 
Effect of buprofezin on spontaneous behavior and impact of atropine
Spontaneous behavior tests were performed in TSD maze for 15 min on adult male rats. Total locomotion activity and rearing mean was measured in all testing rats. Results have shown the rats receiving acute exposure of buprofezin exhibited significant decrease in locomotion and rearing activity and pre-treated atropine rats represent no significant difference compared to control reveals reversal of buprofezin toxicity by atropine. Decrease number of rearing represent the anxiety and depression behavior in buprofezin treated rats, (Figure 7).
Figure 7: A showed the % of correct path choice during acquisition session and reversal of buprofezin induced incorrect path choices by pre-treated
atropine in adult male rats. Pre-treated atropine has shown to increase % of correct path choice. P value *P<0.05, **P<0.01***P<0.001. B represent the
reduced % of correct path choice during navigation on day 1 and its counteraction by pre-treated atropine. Correctness of path selection is increased
with training on day 2. *P<0.05. “a” significant different from control. “b” significant different from pre-treated atropine. 
Buprofezin induced impairment in working memory, reference memory and spatial navigation was counteracted by atropine
Correct choice of path during acquisition career: Our finding has revealed a significant loss of correct path choice during acquisition session in buprofezin exposed rats compared to control. Rats were subjected to five trial for each and percentage of correct choice was calculated. At first day the control group was unable to reach 80% correct choice of path. Second day after continues trial rats ultimately obtained 80% correct choice of path. Impairment of correct path choice was counteracted by pre-treated atropine, (Figure 8A).
Figure 8: A revealed the significant reduction of Working Memory Error (WME) during acquisition session with pre-treated atropine induced by
acute exposure of buprofezin. P value *P<0.05, **P<0.01***P<0. 001. B describe the attenuation of Reference Memory Errors (RME) caused by acute
exposure of buprofezin with pre-administration of atropine. Memory errors reduced continuously from day two to onwards. *P<0.05, ***P<0.001. “a”
significant different from control. “b” significant different from pre-treated atropine. 
Correct choice of path during navigation session: In time space navigation session, the rats were trained to obtained food stimulus from shorter pathway to reach maximum 10% of correct choice although food is baited in both pathways. We have found less than 10% of correct choice of control rats on first day and buprofezin treated rats showed significantly less correctness compared to control. Moreover, pre-treated atropine has reversed the effect. On second day control rats achieved the criteria of 10% correctness after five trials of training. buprofezin again decreased the choice correctness. These finding suggest the deterioration and apoptosis of TSD, grid cells, speed cells and hippocampal place cells following the acute exposure of buprofezin, (Figure 8B).
Working memory and Reference Memory Error during acquisition are attenuated by pre-treated atropine
Working Memory Error: Working memory is memory of object stimulus or recognition of location used in testing session. So if rat enter in food baited path it is working memory correctness and if it reenters into baited pathway when both pathways are baited it is referred as Working Memory Error. On first day of training the Working Memory Errors (WME) were greater in buprofezin treated rats compared to second day. Results suggested that continuous training alleviated the incidence of Working Memory Error and pre-treated atropine significantly attenuated the Working Memory Errors, (Figure 9A).
Figure 9: A revealed the effect of buprofezin and pre-treated atropine on escape latency of rats from one quadrant of maze in visible platform test.
Pre-treated atropine significantly counteracts the buprofezin induced increases in escape latency of rats compared to control. Training decreases the
escape latency from quadrant. P value *P<0.05, **P<0.01, ***P<0.001. B showed the pre-treated atropine significantly reverse the buprofezin induced
increases in escape latency of rats compared to control in hidden platform test. P value *p<0.05, **p<0.01, ***p<0.001. C represent the buprofezin
induced reduction in quadrant search time and its counteraction by pre-treated atropine during probe testing. P value *P<0.05, ***P<0.001. D
represent the buprofezin induced reduction in numbers of platform crossings and its counteraction by pre-treated atropine during probe testing. P
value *P<0.05. “a” significant different from control. “b” significant different from pre-treated atropine. 
Reference Memory Error: Reference memory is memory for rule of given condition. For example, acquisition of baited path provides food to rats. Entries of rat into pathway with no food stimulus are referred as Reference Memory Error. Results suggested that Reference Memory Error (RME) during acquisition session changed days after training. Buprofezin treated group exhibit more Reference Memory Error compared to control group. On second day (RME) were comparatively more from day 2 to onward the memory errors reduced continuously. However, there was no substantial difference found in control and pre-treated atropine group. This demonstrates the reversal effect of pre-treated atropine on Reference Memory Errors, (Figure 9B).
Atropine attenuate the deficit in spatial learning induced by acute exposure of buprofezin
We evaluate the spatial learning of testing rats of all groups using Morris water maze test. In our training procedure animal undergoes four trial of a day. Using spatial cues in room rat were allowed to find visible and hidden platform located in one quadrant of maze. In training process latencies to find platform continuously decreases in treated and control groups. The latency of rats in one quadrant of pool was measured. Results have shown that buprofezin treated rats spend significantly more time in one quadrant while searching platform in all trial represent deficit of spatial learning. However, no significant difference was found between control and pre-treated atropine plus buprofezin, (Figure 10A and 10B).
Figure 10: A demonstrate the decreased hind limb foot distance in buprofezin intoxicated rats. Pre-treated atropine not significantly counteracts the
effect. B significant increase in hind paw lick latency in buprofezin treated rats efficiently reversed by pre-treated atropine. “a” significant different
from control. “b” significant different from pre-treated atropine. 
While probe testing the platform was absent the time spent in searching target quadrant was significantly less in buprofezin treated rats compared to control. However, atropine weakly revers this effect as there was substantial difference between control and pre-treated atropine plus buprofezin.
Similarly, the number of platform crossing was remarkably less in buprofezin exposed group compared to control. Pre-treated atropine strongly counteracts and numbers of crossing were increased near to control as there is no significant difference between control and pre-treated atropine plus buprofezin group. Thus, buprofezin treated rat devoid the spatially selected search strategy and were unable to found the specific location of hidden platform and effect was counteracted by pre-treated atropine. Together these results demonstrate that buprofezin induce various form of hippocampal dependent memory defects as analyzed by fear conditioning, passive avoidance and the Morris water maze.
Hind limb landing foot splay test
Hind limb landing foot splay analysis of all groups was performed on 7th day. Results revealed significant decrease in foot splay in buprofezin treated rats compared to control and no significant difference was found in pre-treated atropine and control rats. The decreased foot splay might be due to skeletal muscle weakness because of peripheral neuropathy, (Figure 11).
Figure 11: Showed the latency of death following the alternative exposure of neostigmine plus atropine in buprofezin intoxicated and non-intoxicated
rats. P value<0.001. Significant latency difference was exhibited by each group. Data was expressed as mean ± SEM. “a” significant different from
control. “b” significant different from pre-treated atropine. 
Hot plat test to evaluate analgesic effect of buprofezin and effect of pre-treated atropine
Hind paw lick latencies were investigated of all groups. Results suggested that rats exposed to acute buprofezin exhibit significant increased latencies to hind paw lick compared to control and there was no difference in pre-treated atropine and control. Buprofezin treated rat’s responses to nociception of heat were lost and were contracted by pre-treated atropine, (Figure 11).
Mechanism of buprofezin neurotoxicity
Buprofezin intoxicated rat died soon after the administration of neostigmine (30 μl/kg/day IP) a blocker of Acetylcholinesterase (AChE). It suggested that a small concentration of AChE in synapse was dominantly occupied by a small concentration of neostigmine consequently tremendously elevated the Acetylcholine (Ach) level in synapse leading to tremor and death of rat. However pretreated atropine delayed convulsion, tremor and death indicated a protective role of pre-treated atropine against buprofezin induced neurotoxicity. Pre-treated neostigmine plus atropine also delayed death due to sufficient availability of AChE in synapse that metabolizes the Ach in non buprofezin intoxicated rats. Pretreated atropine plus neostigmine treatments in non buprofezin intoxicated rats also cause sudden death due to plenty of ACh in synapse as AChE and cholinergic receptors have been blocked.so these results suggested that buprofezin increases the concentration of ACh in synapse by inhibiting the synthesis and release of AChE due insufficient supply of ATP.
The recent study described the neurobehavioral toxicity of acute buprofezin exposure however it is previously well established that buprofezin (BPFN) frequently used as a moulting inhibitor insecticide all over the world to eradicate pests like leafhoppers, mealy bugs and whitefly [24], infiltrating the fruit crops, leafy crops and citrus crops. Its metabolic compounds are potentially hazardous to the neighboring milieu [14]. It has been proved extremely toxic to aquatic environment [11,25] as in embryo of zebra fish Reactive Oxygen Species (ROS) have been detected following the exposure to buprofezin and nickel. Administration of embryos and larvae of African catfish (Clarias gariepinus) to various doses of buprofezin consequences into death of embryos when its amount of dose rises to 5-100 mg/L. In African catfish dose <5 mg/L also carry out numerous hazardous effects during embryogenesis and development of larva. These effects comprise asymmetrical head, bleeding from pericardium, inward curvature of the lumbar and cervical regions, arcuate in body, ulcerates and accumulation of fluid in yolk sac [26].
In our study we have explored that the acute oral dose of buprofezin 87.9 mg/kg/day induced wide range of neurobehavioral toxic effects. Acute intoxication of buprofezin induce a wide range of neurobehavioral toxicity including damage of pyramidal cells of hippocampal CA1 and CA3, region neurons and behavioral impairments for example, loss of motor coordination, locomotor activity, fear loss, hearing, heat shock, sensorimotor, cognitive and spatial navigation impairment following the acute exposure in adult sprague dowley male rats. We have also found that acute intoxication of Buprofezin is potentially reversed by pre-administration of Atropine. The complete molecular and biochemical mechanism of Buprofezin neurobehavioral toxicity is not elucidated so for however we suggested that it inhibit the synthesis and release of AChE in synapse as in our experiment buprofezin intoxicated rat was suddenly dead after the administration of neostigmine (30 μl/kg/day IP) a blocker of AChE. It put forward that a small concentration of AChE in synapse was dominantly occupied by a small concentration of neostigmine consequently tremendously elevated the ACh level in synapse leading to tremor and death of rats. This hypothesis was also supported by previous studies as activity of cytochrome and TCA cycle enzyme was rendered by buprofezin that interfere the energy metabolism and inhibit production of ATP [15]. Atropine function as a physiological antagonist and competitively block the Acetylcholine action of muscarinic receptors and acts as antidote for excessive parasympathetic activation arised as consequence of inhibition of AChE [27]. Acetylcholinesterase (AChE) being serine hydrolase enzyme that cleaves rapidly terminate the cholinergic transmission in synapse by breakdown of Ach into choline and acetate [28]. AChE also described as key player in activation of glial cells, brain blood flow, amyloid pathway, phosphorylation of tau protein, also function as adhesion protein for maintenance and development of synapse [29]. Arsenic exposure in animal model induces behavioral alteration, abnormality in nervous system shaping and development, inflammation and neuron death, [30,31]. In addition, arsenic could induce toxicity in Highly Aggressive Proliferating Immortalized (HAPI) microglia [32], granular neurons of cerebellum [33] and snail neurons [34].
Further our study demonstrated the impairment of motor coordination and fore limb and hind limb grip strength as Occupational exposure to acrylamide leads to cumulative but reversible neurotoxicity described by axanopathy of peripheral nerves, ataxia, muscle weakness, tingling of hands and feet and cognitive deficiency. Because it inhibits kinesin transport, decrease the neurotransmitters and inhibition of transmission [35]. Crofton and colleagues stated decreased grip strength in a 30-day acrylamide administration study at 15 and 20 mg/kg/ days because it causes peripheral axonopathy [36] after exposure to high oral doses of buprofezin clinical signs appear as reduced locomotor activity, tremble, runny nose, abnormal movement and urinary discontinuous urination [14]. In present study impairment in the grip strength and motor coordination was assessed by using rotarod, horizontal and parallel bars. Similar impairments in motor activity, abnormal gait, and cognitive deficiency were detected following exposure to bifenthrin due to oxidative stress [37]. A study on earthworm described that reduction in growth rate by combined exposure of buprofezin, lufenuron, and triflumuron pesticide-exposed worms was observed by dose-dependent over the 28-day treated duration, which was accompanied by a decline in activity of AChE and GST. The lowest activity of AChE was noted at the highest dose of buprofezin (300 mg/kg soil) following two weeks of exposure as compared to control. The activity of AChE was intensely repressed by lufenuron subsequently by buprofezin, and then triflumuron in descendant. [16]. Similarly, our study showed that acute exposure of buprofezin inhibit the synthesis and release of AChE in synapse due to limited supply of ATP described by other study that buprofezin efficiently repressed the cytochrome c oxidase activity by binding to SCO1 active pockets and COX17, which increased the concentration of reactive oxygen species. Additionally, administration with an ROS inhibitor (N-Acetyl-L-cysteine) (NAC) counteracts the decreased level of ATP and cytochrome c oxidase activity, which also showed that ROS contributed in buprofezin-induced conversion of energy metabolism. After sub lethal treatment of buprofezin, the levels of the end product metabolism (ATP), end product of glycolysis (lactate) and (pyruvate) a component in initial stage of the TCA cycle were evaluated. The higher concentration of these factors after buprofezin exposure reveals the BPFN induced inhibition of TCA cycle. Pesticides can decrease the ATP concentration in HepG2 cells revealed by in vivo and in vitro studies [38,39].
Pre-treated atropine counteracts the poisoning by blocking muscarinic receptors and it counteracted over parasympathetic activity. At single oral dose (24 h) of chlorpyrifos reduced the activity of plasma Butyrylcholinesterase (BChE) and rats (AChE) activity in hippocampus, striatum and prefrontal cortex. The acute chlorpyrifos toxicity can be counteracted by the atropine antidote (10 mg/kg IP) and/or pralidoxime (40 mg/kg; IP) treated one hour following toxicity [40]. In pre-clinical and clinical experiments muscarinic antagonists exhibit antidepressant effects [41-43]. Acute buprofezin decreases the synthesis and release of AChE in synapse. Furthermore (IP) neostigmine injection in buprofezin intoxicated rats cause tremor, and sudden death of rat also proposed that minor amount of AChE is predominately blocked by neostigmine extremely elevated the ACh level in synapse subsequent acute exposure and is counteracted by pre-treated atropine.
Buprofezin exposure impair the passive avoidance as MSK1 knockout affects numerous various forms of hippocampusdependent memory, as evaluated by fear conditioning, Morris water maze and passive avoidance [44]. Pre-treated atropine showed counteract effect not reported in any previous study.
The behavioral analysis revealed that SA (Sodium Arsenide) treatment cause loss in learning and memory in passive avoidance as well as motor activity and balance. Additionally, acute or chronic administration to SA as revealed by other experiments induce abnormalities of CNS containing slowing of cognitive development, decreased psychomotor speed, loss of learning due to decreased number and apoptosis of pyramidal cells and was mitigated by ellagic acid [44-46]. Our finding also agreed with similar behavior abnormalities and loss of passive avoidance learning and memory due to pyramidal neuron damage in hippocampus and was attenuated by pre-treated atropine. Buprofezin induced decreased in the step-through latency was reversed by pre-treated atropine as the SA exposure has showed significant reduction in the step-through latency comparison to the control due to oxidative stress [47]. The study reported that MSK1 knock-out animals can process the sensory information of foot shock and memorize it in association with contextual and the auditory stimulus, but loss this memory in 24 h [47]. Our study reported the impairment in long tern contextual fear memory and rapid loss of sensory stimulus of foot shock in acute buprofezin exposure and was slightly counteracted by pre-treated atropine.
Various concentration of orally administered imidacloprid to female rats caused a substantial change in different features of locomotor activity and decreased in ambulation at 90 days of treatment as it inhibits AChE activity [48]. Substantial reduction in locomotion in the rats exposed with the acute dose of imidacloprid has suggested that imidacloprid or its metabolic residues has accumulated in brain. Administration of imidacloprid directly in to intraperitonium has shown to accumulated in mouse brain [49].
Although the mechanism of action of buprofezin is distinct to some extant results described the similar decline in spontaneous locomotion activity in acute buprofezin administered rats compared to control and pre-treated atropine counteract the toxicity. Similar to high dose of imadacloprid decreases the spontaneous locomotor activity. Another study also supported our results that during the peak of the BGS (Brain Growth Spurt) (PND10) administration of single dose of endosulfan or cypermethrin, cause long lasting spontaneous behavior abnormality in adults due to alteration of protein involved in brain development, variation in locomotion, rearing and total activity without affecting body weight as compared to control mice [50].
It has also been stated that the number of hippocampal neurons declined in rats after treatment with sulfite [51]. Additionally, it was revealed that after sulfite exposure the excitability of the spinal reflexes was increased [52,53]. The toxic consequences of sulfite on mesencephalic cell lines have been described, as well [54].
Our study in line with these findings as pre-treated atropine has neuroprotective effect against buprofezin toxicity. Pre-treated atropine prevents hippocampal neuron degeneration, spatial memory impairment, working and reference memory loss by preventing over excitability induced neuron exhaustion and preventing the apoptosis of hippocampal neurons. It also prevents the oxidative stress of hippocampal neurons and protect against buprofezin induced cognitive impairment as curcumin inhibit lead-induced loss of memory in rats [55]. Curcumin can alleviate the cognitive deficit in diabetic rats [56]. Therefore, curcumin may preclude the oxidative stress in CA neurons and, as result, may enhance the synaptic plasticity [56,57]. It is also stated that curcumin protect the neurodegeneration in parkinson and alzheimer disease [58]. Additionally, it is also suggested that curcumin prevent the apoptosis of neuron [59].
However, an impairment of spatial memory ability was observed in 1-month-rotenone exposed group model animal. Later it was well acknowledged that the hippocampus was indispensable for spatial learning and memory performance on Morris water maze task [60,61]. The previous study described that (3.5 mg kg-1 and 7 mg kg-1) doses of bifenthrin caused remarkable spatial memory deficit of the rats hence the quadrant spending time was significantly less compared to control [38]. In contrary in our findings buprofezin intoxicated rats exhibit increased escape latency compared to control. Additionally, quadrant search time and numbers of platform crossings were decreased compared to control.
Yet, another study described Hind limb foot splay of both males and females assessed on the day 15 exhibited statistically significant increase in male rats exposed with acrylamide at 40 mg/kg body weight as compared to control and showed no effect on female. In an earlier study, acrylamide at 40 mg/kg represented a biologically substantial increase in foot splay [62]. In contrary our study revealed the significant decrease in hind limb foot splay distance in acute buprofezin treated rats compared to control and pre-treated atropine counteract the effect. These finding suggested that pretreated atropine attenuate the peripheral neuropathy caused by acute exposure of buprofezin.
Our results also demonstrate that acute exposure of buprofezin produce anti nociceptive effect and is reversed by pre-treated atropine as the treatment of PC/β-CD (P cymen and beta cyclodextrin) in hot-plate test, for all doses, remarkably decreased the sense of nociception for 8 h while only PC for 1 h, occur only at high doses likely caused by motor abnormality [63].
The finding of our study reported that acute oral administration of buprofezin cause weight loss and decrease the food consumption. A small weight gain was noticed in pre-treated atropine rats as demonstrated that oral administration of buprofezin 73.97 mg/kg bw/day in males and 93.11 mg/kg bw/day in females enhance the weight of organs and decreased the body weight gain [64].
A wide range of effects produced by Trichlorethane (TCE), and ether are similar to ethanol and depressant like properties of volatile solvent described in earlier literature [23]. these involved decreases in activity of CNS (alterations in posture. reduced arousal and rearing), decreases in emotionality of CNS (increased ease of removal), impair of muscle tone (disturbances in gait reduction in forelimb grip strength, increased landing foot splay and loss of psychomotor coordination on the inverted screen test), and decreased sensorimotor activity (reduced response to sensory stimuli). Though TCE and ether at concentrations of 13.300 and 30.000 ppm substantially increased landing foot splay. Flurothyl exposure did not affect increase in landing foot splay at any concentration. TCE and ether also decrease forelimb grip strength but not flurothyl. Additionally, flurothyl produccd handling-induced tremors after animals was removed from cage, effect which was not caused by TCE, ether or ethanol. Flurothyl cause postictal depression (e.g., an increased inversion latency on the inverted screen test) Characterized by convulsion in testing animal and exhibit not any ethanol like properties [65]. The results of our study also agreed with these findings as the acute exposure of buprofezin in rat results in decreased brain activity, posture abnormality, decreased activity level, increased lacrimation, salivation, piloerection, abnormal muscle tone, loss of limb grip, ambulation and gait abnormalities, decreased rearing and arousal, loss of time space navigation ability, loss of sensorimotor responses, Redness of nostrils, muscle hypoplasia, decrease in body weight and body temperature. However pre-treated atropine attenuated these variations and significantly reversed these effects.
Parallel to our results previous studies documented the body weight of rats exposed to acute and subacute lambda cyhalothrin was significantly decreased and was observed that green tea extract moderate the body weight loss.The decline in body weight was consequent of decrease appetite and food consumption as the pesticides effects the digestive tract [68]. Pyrethroids exposure has been described a cause of body weight loss [66,67]. In contrast to our studies, the brain weight of rats following acute and subacute lambda cyhalothrin exposure was increased as concomitant synthesis and accumulation of lipids and cholesterol in brain [68].
The farmer studies have various shortcoming as they devoid acute buprofezin neurotoxicity in rat model. Additionally, none of previous study has reported the mechanism underlying its neurotoxicity in rats. Further no therapeutic strategy against buprofezin toxicity was carried out. However, the study has some limitation as for clear interpretation of neuronal toxicity, advanced immunohistochemistry, confocal imaging and Terminal Deoxynucleotidyl Transferase DUTP Nick End Labeling (TUNEL) assay, ROS assay using DCFH-DA, for apoptosis are required. In order to reveal molecular mechanism of neurobehavioral toxicity, molecular and genome sequences techniques mandatory. Pretreated atropine showed significant effect against buprofezin toxicity, however further studies are required to revealed the molecular mechanism of buprofezin toxicity and ameliorative effect of post-treated atropine antidote. In contrary our finding has proved that acute exposure of buprofezin induced neurobehavioral toxicity in adult sprague dowely male rats has been potentially recovered by pre-treated atropine.
Conclusively our findings have explored that acute exposure of buprofezin in rats induces a profile of neurobehavioral toxicities potentially attenuated by pre-treated atropine antidote. Moreover, its neurobehavioral toxicity was induced by impairment of synthesis and release of Acetylcholinesterase (AChE) in synapse. Further studies are necessary to validate molecular and biochemical mechanism involved in decreased concentration of AChE in synapse.
The authors are thankful to Faculty of Biological Sciences, Quaidi- Azam University, Islamabad Pakistan and NIH for providing Rats for experimental purpose.
We have no competing interests to disclose.
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Aslam M (2022) Atropine Reverts the Neurobehavioral Toxicity Elicited by Acute Exposure to Buprofezin in Sprague Dowley Rats. J Clin Toxicol. 12:506.
Received: 30-Mar-2022, Manuscript No. JCT-22-16466; Editor assigned: 01-Apr-2022, Pre QC No. JCT-22-16466 (PQ); Reviewed: 15-Apr-2022, QC No. JCT-22-16466; Revised: 22-Apr-2022, Manuscript No. JCT-22-16466 (R); Published: 02-May-2022 , DOI: DOI: 10.35248/2161-0495.22.12.506
Copyright: © 2022 Aslam M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.