
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Research Article - (2018) Volume 7, Issue 4
Malaria remains an endemic infection which results in nutritional deficiencies. This study was set up to assess the antioxidant capacity and the levels of some selected minerals in the plasma samples of malaria infected individuals. Blood specimens were obtained from ten P. falciparum infected patients and ten healthy individuals using standard clinical procedures. Plasma levels of superoxide dismutase, catalase and glutathione were determined using standard spectrophotometry methods. Plasma levels of the following electrolytes; calcium, sodium, magnesium and potassium were determined using standard atomic absorption spectrometry methods: Results show that there was a significant (p<0.01) decrease in the plasma levels of SOD, CAT and GSH, calcium, magnesium and sodium of P. falciparum infected individuals as compared to P. falciparum free individuals with no significant (p> 0.01) difference in the serum levels of potassium for both. The presence of malaria parasites ( P. falciparum) in blood affects the activities of antioxidant enzymes and micro nutrients in patients.
Keywords: Malaria; Micro nutrients; Antioxidant; Health
Malaria is a prevalent, endemic disease in tropical and subtropical regions of Africa affecting about 300 to 500 million people a year and is responsible for about 90% morbidity and mortality [1-3]. Malaria parasites are carried by mosquitoes and there are different types of malaria parasites of which the commonest is Plasmodium falciparum. Malaria infection is a febrile illness responsible for 300 to 500 million clinical cases annually [4].
Free radicals; also known as reactive oxygen species (ROS) e.g O2-, H2O2 are generated endogenously in the body and have been linked to the progression of most diseases including malaria. These radicals are very reactive and react with biomolecules in the cell resulting in an alteration in the intracellular and intercellular homeostatic balance [5]. There also exists in the body a ubiquitous antioxidant defence system which include superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH) and other antioxidants like ascorbate, α-tocopherol, thioredoxin, vitamins etc., which help to counteract the damaging effects of these reactive oxygen species [6]. However, an imbalance between the antioxidant defence system and ROS production does occur when there is an overwhelming production of ROS which consequently results in a condition known as oxidative stress [7].
Malaria infection has been known to result in the generation of hydroxyl ions radicals in the liver which creates oxidative imbalance [8]. Moreover, the complications associated with the clinical state of malaria can become compounded if there is an imbalance in the electrolyte levels in the body. There is still a relatively existing knowledge gap in the pathogenesis of malaria disease and hence this study was targeted at investigating and bridging some of the knowledge gaps that exist in understanding the effects of Plasmodium falciparum on the antioxidant status and levels of selected electrolytes (calcium, magnesium, potassium and sodium) in malaria patients as this will provide more insight on the clinical management of malaria infection.
Subject collection
Blood samples from ten clinically confirmed P. falciparum malaria patients and ten healthy individuals were used for this study. Prior to blood collection, informed consent and ethical approval was obtained. The blood specimens were collected into ethylene diamine tetra acetic acid (EDTA) bottles and centrifuged at 4500 rpm for five minutes in order to separate the plasma from whole blood.
Analysis of antioxidant parameters
Catalase: Catalase activities of the samples were determined in erythrocyte lysate using Aebi’s method [9]. To a cuvette containing 450 μL of phosphate buffer (0.1M, pH 7.4) and 500 μL of 20 mM hydrogen peroxide was added 50 μL of the lysate. Activity of catalase activity was measured at 240 nm for 1 minute with a spectrophotometer. The molar extinction coefficient of hydrogen peroxide, 43.6 Mcm-1 was used to determine the catalase activity. One unit of activity is equivalent to 1 mMol of hydrogen peroxide decomposed per minute and was expressed as units per milligram of protein.
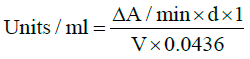
d=dilution of original sample for catalase reaction, V=sample volume in catalase reaction (ml), 0.0436=ε^mM and 1=total reaction volume
Superoxide dismutase (SOD): This was determined in accordance with the method described by Mccord and Fridovich [10]. To 20 μL of the lysate, 250 μL of 75 mM Tris-HCl buffer (pH 8.2), 30 mM EDTA and 30 μL of 2 mM pyrogallol were added. A spectrophotometer was used to read the increase in absorbance recorded at 420 nm for 3 minutes. One unit of enzyme activity is equivalent to 50% inhibition of the rate of auto-oxidation of pyrogallol as determined by change in absorbance/min at 420 nm. The activity of SOD was expressed as units/ mg protein.
Rise in absorbance per minute = A3− A0 /2.5
where A0=absorbance after 30 seconds
Glutathione: Reduced glutathione (GSH) was measured using the method of Sedlak and Lindsay [11]. To 1 ml of the supernatant was added 0.5 ml of 10 mM Ellman’s reagent and 2 ml of phosphate buffer (0.2 M, pH 8.0). A spectrophotometer was used to measure the intensity of the yellow color observed at 412 nm with a blank containing 3.5 ml of phosphate buffer. A series of standards were also treated similarly. The amount of GSH was expressed in mg/100 g of tissue.
Mineral analysis by flame atomization
The levels of calcium, potassium, sodium and magnesium in all the plasma samples were determined using the calibrated Perkin Elmer Analyst 400 atomic absorption spectrometer. Small quantities of the samples were aspirated into the flame where the ions were reduced to elements and vaporised. A light beam with a single specific wavelength for the element being measured was directed through the flame and was detected by a monochromator.
Statistical analysis
The data obtained from the analysis of plasma antioxidant parameters and electrolytes level was statistically compared for the two groups. Student’s t-test was used to check for significant difference (99% confidence interval).
Antioxidant parameters (SOD, CAT, GSH) in P. falciparum infected and free plasma samples
The result of the SOD, CAT and GSH levels in the plasma samples is presented in Table 1. The result revealed that P. falciparum free plasma samples contained a significantly higher (p<0.01) amounts of SOD, CAT and GSH than the P. falciparum infected samples.
| Sample | GSH (µmol/ml/min) | SOD (µmol/ml/min) | CAT (µmol/ml/min) |
|---|---|---|---|
| P. falciparum infected plasma |
1.20 ± 0.08a | O.78 ± 0.65b | 1.58 ± 0.61a |
| P. falciparum free plasma | 1.84 ± 0.04b | 6.11 ± 1.38a | 3.11 ± 1.78b |
Table 1: Levels of antioxidant parameters (GSH, SOD, CAT) in P. falciparum infected and free plasma samples. Values are Mean ± S.D (n=10); a, b Means are significantly different from each other (p<0.01).
Levels of electrolytes (Ca, Mg, K, Na) in P. falciparum infected and free plasma samples
As presented in Table 2, there was a significant (p< 0.01) decrease in the plasma levels of calcium, magnesium and sodium in the P. falciparum infected samples as compared to the P. falciparum free samples. However, no significant difference was observed in the level of potassium in both.
| Sample | Ca (mol/l) | Mg (mol/l) | K (mol/l) | Na (mol/l) |
|---|---|---|---|---|
| P. falciparum infected | 0.52 ± 0.08b | 3.53 ± 0.54b | 4.73 ± 2.30b | 79.75 ± 23.50b |
| P. falciparum free | 0.56 ± 0.07a | 3.68 ± 0.61a | 4.73 ± 2.30b | 83.88 ± 14.55a |
Table 2: Serum levels of Ca, Mg, K and Na in P. falciparum infected and free plasma samples. Values are Mean ± S.D; n=10; a, b Means are significantly different from each other (p<0.01).
In human plasma and other extracellular fluids, antioxidants participate in defence against oxidative damage. According to Asif, antioxidant enzymes act against free radicals; however, oxidative stress occurs when free radicals are generated in amounts greater than the scavenging capacity of the endogenous antioxidant system [12]. Oxidative stress has been linked to the pathogenesis of most diseases including malaria. Earlier reports have implicated plasmodium parasites in the induction of oxidative stress during its erythrocytic stages [13]. Reactive oxygen species (ROS) are formed in the host as a consequence of the breakdown of haemoglobin by the parasite in which the host tries to counter these by producing antioxidant enzymes like SOD, GSH, and catalase to mop out some of these reactive oxygen species [13]. The ROS generated in host-parasite interactions can cause several biochemical changes including lysis of red blood cells and alteration in the amounts of major antioxidants present in those red blood cells [8].
Our results showed that there was a significant (p < 0.01) reduction in the level of the three antioxidant indices – GSH, SOD and CAT in the P. falciparum infected plasma samples as compared to the P. falciparum free samples. The reduced GSH, SOD and CAT level in the research is in complete agreement to the work of Rodrigue et al. and Oyewole et al. who also recorded a significant (p< 0.05) reduction in the levels of GSH, CAT and SOD in high parasitaemic patients [14,15]. The reduced GSH, SOD and CAT levels obtained in this study could be due to the high P. falciparum counts in the blood of the infected patients. However, Ramya suggested that elevated antioxidant enzymes level in patients with reduced number of parasites might be attributed to the reaction of the host defensive system to the surge in reactive oxygen species caused by P. falciparum or its metabolites [16]. Plasmodia erythrocyte cells accrue protective antioxidant parameters such as catalase, glutathione peroxidase and superoxide dismutase which are depleted in the infected host [17]. This might be for the purpose of exploiting erythrocyte proteins for metabolic requirements as the parasite develops. Patients with high parasite count might be susceptible to higher oxidative stress causing damage to erythrocyte membranes which also leads to reduction in the deformability of the cells, signaling the removal and substantial eradication of erythrocytes by macrophages. These subsequently result in very severe anaemia, constriction of peripheral microvasculature, hypoxia of the cerebrum, and cardiac damage that are included among the characteristics of severe malaria [17,18].
The minerals existing in blood and other body fluids are referred to as electrolytes. These minerals are required to be present in an optimum range as this is important for normal physiological activities of the human body. Among the symptoms of a number of infectious diseases including malaria are electrolyte and mineral imbalances. Plasmodium parasites usually induce hyponatraemia, hyperkalaemia, hypocalcaemia and hypomagnesaemia in infected individuals [19].
The extracellular fluid has sodium (Na) as its major constituent. Sodium controls the normal circulation of water and osmotic pressure in several body fluids. A disruption in the level of sodium ion results in various health problems [20]. Hyponatraemia which is a reduction in the sodium ion concentration is regarded as a key clinical index of malaria. Hyponatraemia amplifies the symptoms of malaria making it austere [21]. Result from this study revealed that the plasma level of sodium in the P. falciparum infected individuals was significantly (p <0.01) lesser than that of the P. falciparum free counterpart. The underlying mechanism behind reduced sodium level in malaria remains evasive, but various studies have reportedly linked an upsurge in the secretion of vasopressin (ADH) to the hyponatraemia observed in malaria, due to the fact that sodium may move into the infected cells and cause loss of blood [22]. This has been corroborated by results obtained from previous researches [23,24].
Potassium (K) is also a vital electrolyte in the human body as it directly has an effect on the heart muscle cells. It is necessary for the normal working of nervous system and heart muscle activity. Weakness, fatigue and rapid heartbeat might result when there are small changes in the potassium level. Hence, for the human body to function optimally under physiologic conditions, potassium balance is crucial [25]. However, there was no significant difference in the levels of potassium obtained for both P. falciparum infected and free plasma samples and this finding is in alignment with a study done by Maitland et al. who also observed no change in the level of potassium in patients suffering from malaria as compared to healthy individuals [26]. This is however contrary to the results obtained from previous studies by Yoel and Ikekpeazu et al. who reported a decline in the potassium levels of malarial patients [27,28].
The mineral present in the greatest amounts in the body is calcium and is stored majorly in the bones. It is necessary for formation and building up of teeth and bones. Calcium is also involved in other regulatory functions in the body system [25]. Variations in calcium level can result in numerous changes in the body like muscle spasms, osteoporosis etc., [29]. A decline in the level of plasma calcium was observed in the P. falciparum infected samples compared to the P. falciparum free samples. This is in alignment with the report given by Ayoola et al. who also observed a diminution in the level of calcium in malarial patients [30]. The observed decline in calcium level could be due to trophozoites that accumulate calcium in their internal compartment for metabolism as suggested by Gazarini et al. [31]. The losses in calcium can likewise be triggered as a result of digestive and renal complications arising due to malaria. Infection by Plasmodium parasites may also alter the absorption of cells for calcium according to Tiffert et al. [32]. Infected cells amplified permeability of calcium, which resulted in low levels of calcium in the blood of malarial patients. Another likely reason for low calcium levels in the blood (hypocalcaemia) is that parasites may stick to the glomerular capillaries triggering renal inefficiency which might lead to greater urinary excretion of minerals like calcium.
Magnesium (Mg) has been reported to be a cofactor involved in more than three hundred enzyme systems that controls the diverse biochemical reactions in the human body. It plays a role in muscle and nerve functions and also in the regulation of blood glucose and blood pressure [33]. Magnesium is needed in a balanced amount for the proper functioning of the body. Its shortage causes retching, lethargy, weakness, muscle contraction and spasms [33]. The present study revealed a significant (p<0.01) decrease in the magnesium level of the P. falciparum infected blood samples as compared to the P. falciparum free sample. This is however contrary to the result obtained in a study by Garba who observed an increase in the plasma magnesium level of malaria patients suffering from P. falciparum parasite [34]. This disparity might be due to the different parasite counts and stages presented by the patients used for the study.
In conclusion, infection with P. falciparum malaria parasite leads to a slight reduction in the plasma levels of antioxidant enzymes and some electrolytes in infected individuals. This could lead to complications that can further predispose the infected individuals to other malnutrition-induced diseases. A co-supplementation with some of these electrolytes and antioxidants is therefore recommended during the course of treating the disease. Moreover, a further research is recommended to study the effect of other types of malarial parasites on other biochemical and physiological parameters in humans.