
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2022)Volume 12, Issue 2
Sulphites like Sodium Metabisulphite are commonly used as preservatives in food items, beverages, cosmetics and pharmaceuticals. Data about the adverse effects of such chemicals is missing in regards to infants and children’s. The aim of current study was to evaluate the effect of a Sodium Metabisulphite on prepubertal rats as analogue model to human child. Thirty-two prepubertal rats (28-30 postnatal days) were divided into four groups (8/group). They received distilled water, Sodium Metabisulphite (75 mg/kg/day), vitamin C (20 mg/ kg/day) and sodium metabisulphite+vitamin C. All the animals received daily gavages for 4 weeks. Parameters included body weight gain, hematology, serum chemistry, oxidative stress markers and antioxidant level in serum. Mean body weight gain was markedly decreased. Complete blood count, liver function test (AST, ALT and ALP), kidney function test (creatinine and urea) oxidative stress biomarkers (TBARS and ROS) and antioxidant enzymes level (SOD, POD, CAT and GSH) showed significantly pathophysiological hallmarks caused by Sodium Metabisulphite and were ameliorated by the vitamin C. The present study has demonstrated adverse effect of food additives like Sodium Metabisulphite exposure at neonatal life can induce profound toxic effects in rodents.
Sodium Metabisulphite (SMBS); Antioxidant; Food additives; AST; SOD; Vitamin C
Food additives are used for preservation, sweetening and colouring of food. The freshness of food cannot be maintained for a long period as decomposition or spoilage begins very soon. Preservatives constrain deterioration and spoilage of food which usually occurs due to the availability and growth of microorganisms and the oxidation activity of bacteria and yeast. Certain additives are added in ice cream, jams, biscuit and making bread to enhance the taste of edibles [1]. Food additives are useful in many ways such as salt being used as a preservative and flavouring agent [2]. Food additives on the other hand have some adverse effects. Benzoic acid is a food additive and damages the central nervous system, makes the consumer suffer from hypertension, asthma and hyperactivity in the children [3].
Recent evidences indicated that many foods preservatives, additives, colorings, and packaging materials have adverted effects on children's health according to new American Academy of Paediatrics (AAP) policy statement who has calls for immediately betterments in the U.S. food additive regulatory processes [4]. Data about the adverse effect of food additives on infants and children are very limited, but in general, infants and children are relatively more vulnerable to such kind of chemicals (due to higher dietary intake per pound) and additionally the reality that their fundamental organs are still changing as well as being maturing [5]. Sulphites are a group of sulphur-based compounds that potentially release Sulphur Dioxide (SO2), an active component that helps to preserve food [6,7]. Sulphites have been added to foods as preservative agents and other purposes for centuries [6]. Additionally, sulphites are used in the pharmaceutical and cosmetics industries as preservatives of the active ingredient [8]. Sulphites are very efficient and used in several commonly used products, but now their use has been restricted these days due to potential toxic effects [9]. Proteins and lipids may also react with these radicals [10].
Behavioral changes and clinical signs such as anorexia, hair loss and diarrhea were observed when rats were administered high doses of sodium metbisulphite (4%) to evaluate the subchronic toxicity. Their toxic effects that are attributed to the release of sulphur and oxygen free radicals can damage the central nervous system [10-12]. In a study conducted by Reist, et al. [13], showed that sulphite exerts toxic effects on neuronal cells grown directly or in combination with peroxynitrite.
Administration of SMBS in a concentration of 1% and 4% showed a significant effect on the body weight gain, water intake, significant decrease occurred in the biochemical parameters e.g., urea, creatinine, uric acid, calcium and transaminase and decrease in immunoglobulin level. Physiological aspect showed an enlargement in kidney, spleen, liver, and stomach when exposed to subchronic intake of SMBS 1% and 4% [14].
SMBS potentially reduces the dose dependent cell viabilities in similar concentration found in some dried fruits. SMBS induces cell apoptosis and apoptosis associated genes revealed its mechanism of cytotoxicity [15]. SMB has documented as cell deteriorative food additive as it mediate apoptosis in Human Fetal Foreskin Fibroblasts (HFFF2) cells in dose dependent manner. Also reported a higher level of pro-apoptotic genes, Bax, caspase 8, and caspase 9 and decreased expression of Bcl-2 following the cells treated SMB [16,17]. Additionally, histological evaluation showed enlarged, spleen white pup, inflammation of spleen and gastric mucosa and alteration in hematological parameters of wistar rats administered with 4% of NaMBS dose [18]. All these food additives generate free radicals or ROS which induces genotoxic, cytotoxic and mutagenic effects [19]. Sodium bisulphite, a common ingredient of beverage like sodas and fruit juice products produces carcinogens [20,21].
Vitamin C
Vitamin C (ascorbic acid) is a required nutrient for a variety of biological functions. The health-promoting effects of vitamin C can be attributed to its biological functions as a cofactor for a several enzymes, most notably hydroxylases involved in collagen synthesis, and as a water soluble antioxidant. Exhibited significant role in cancer chemo-prevention in the treatment of cancer, sepsis, and neurodegenerative diseases [22-25]. Ascorbic acid, (vitamin C) is a water soluble vitamin, effective in scavenging free radicals, including hydroxyl radicals, aqueous peroxyl radicals and superoxide anions [26]. Moreover, the antioxidant potential of ascorbic acid is not only attributed to its ability to quench reactive oxygen species, but also to its ability to regenerate other small antioxidant molecules, such as tocopherol, glutathione and carotene [27-29].
As none of previous study has been performed to investigate adverse effects of sulphites in pre-pubertal rodent model and what is the role of vitamin C in preventing its toxicity. The present in vivo study investigated the role of vitamin C against Sodium Metabisulphite induced toxicity in laboratory rat in vivo. Study has aims to investigate the effects of SMBS on haematological, biochemical, and antioxidants status in neonatal rodents. It also investigated the effect of sulphites on the growing age of rat as they are probably more vulnerable to be affected by such kinds of toxins because of their high food intake as well as metabolic rate.
Animals and maintenance
Healthy and uniform size male pre-pubertal male rats having age between 28-30 postnatal day and having body weights 80.0 g ± 10.0 g. The experiments were accompanied in accordance with the guideline of animal research ethics committee in Turkey and ethical approval was granted by the KOC university animal research ethics committee. Animals were restrained into clean rodent cages to avoid any contamination. Animals were acclimated under standard laboratory conditions for two weeks before the start of experiment. Distilled water and standard rodent diet was provided ad libitum. Animal room was maintained at temperature variation of 25°C ± 2°C, a photoperiod of 12:12 h, dark: Light cycles and room humidity of 50%–60%.
Experimental design and procedure
The study was designed to evaluate the effect SMBS on physiological, biochemical and serum stress biomarker in prepubertal rat and protective effect of vitamin C. Prepubertal rats were selected as model because of growing age of animal is more susceptible to toxic effect of these chemicals. SMBS LD 50 was calculated through trials that was 2091 mg/kg. This LD 50 was divided into experimental period (28 days) that was 75 mg/kg/ day for subchronic study. Four groups of rats (8/group) received distilled water, Sodium Metabisulphite (75 mg/kg/day), vitamin C (20 mg/kg/day), sodium metabisulphite+vitamin, administered through oral gavage for consecutive 28 days.
Collection of blood samples
On day 29 after the completion of desired period i.e., 24 hr of last dose, animals were anesthetized with a single intraperitoneal injection of ketamax dose of 20 mg/kg. Blood (3 ml) was collected directly from the heart in the EDTA.K3 coated vacutainers (EDTA. K3, BOLTON, scientific limited),) for complete blood count. For serum analysis 5 ml blood was collected in non-heparinized syringes and transferred to gel tube (BIOTUBE BT, vacuum tube sterile, gel clot activator) and kept at room temperature to get clot for about 30 min. Serum separation was done on a bench top centrifuge at 3500 rpm for 15 min. Serum was collected in autoclaved eppendorf tubes with the help of micropipette and stored at -20°C until analyzed. ALT, AST, ALP, urea and creatinine were analyzed with commercial kits on a UV-Visible spectrophotometer (Agilent 8453, California, USA).
Complete blood count by haematology analyser
Blood that collected in EDTA vacutainers was immediately subjected complete blood count that was performed by hematology analyser (Bakeman USA). Blood count provided estimation of hemoglobin, Red Blood Cell Count, RBC Distribution Width (RDW), White Blood Cells Count (TLC), absolute leukocyte count (neutrophils, eosinophils, monocytes and lymphocytes), hematocrit, Mean Crepuscular Volume (MCV), Mean Crepuscular Hemoglobin (MCH), Mean Crepuscular Haemoglobin Concentration (MCHC), platelet count and mean platelet volume in the sample blood.
Serum biochemical parameters
Serum samples were analyzed for the biochemical parameters, renal and liver function tests, total cholesterol and triglycerides concentrations through commercial kits on a UV-VIS Spectrophotometer (Agilent 8453, California, USA).
To determine the enzymatic activities of Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) and Alkaline Phosphate (ALP) commercial kits were used. For determination of urea and creatinine, and uric acid concentration commercial kit’s protocols were followed. All enzymatic activities were measured at 37°C and the results were presented in respective units according the kits.
Protein estimation
Protein concentrations were determined according to the Bradford method using Bovine Serum Albumin (BSA) as standard.
Oxidative stress profile
Reactive Oxygen Species (ROS) assay: Reagent 1 (R1) was prepared by mixing 10 mg of N, N-Diethyl Para Phenyl Diamine (DEPPD) sulfate in 100 mL of distilled water while reagent 2 (R2) was prepared by adding 50 mL of stock solution of ferrous sulfate (FeSO4) in 100 mL of sodium acetate buffer. Stock solution of ferrous sulfate (FeSO4) was prepared by dissolving 50 mg of ferrous sulfate in 10 mL of sodium acetate buffer (pH 4.8). R1 and R2 were mixed at the ratio of 1:25 and kept in dark for 2 min. Working reagents (1680 mL), buffer (1200 mL) and homogenate of sample (60 mL) were taken in a cuvette. Absorbance was measured at 505 nm three readings were taken at intervals of 15 s for each sample and averaged.
Lipid Peroxidation (TBARS) assay: Reaction mixture for Thiobarbituric Acid Reactive Substances (TBARS) was prepared by adding 150 mM Tris-HCl (0.1 mL), 1.5 mM ascorbic acid (0.1 mL), distilled water (0.6 mL) and homogenate (0.1 mL) in a test tube. The mixture was vortexed and incubated in an incubator at 37°C for 15 min. Trichloroacetic Acid (10% TCA, 1 mL) and 0.375% Thiobarbituric Acid (TBA, 1 mL) were then added into the reaction mixture, mixed and boiled at 90°C for 15 min in a water bath. Reaction mixture was centrifuged at 926 g for 10 min and supernatant was collected. Absorbance of supernatant was measured at 532 nm three times for each sample to take the average value.
Superoxide Dismutase (SOD) assay: For the determination of SOD, 1.5 mL L-methionine, 1 mL Nitroblue Tetrazolium (NBT. 2HCl) and 0.75 mL Triton X-100 were mixed, volume was raised to 30 mL by adding Phosphate Buffer Saline (PBS); 1 mL of this solution was then mixed with 20 mL homogenate. This mixture was illuminated for 8 min to fluorescent light and incubated for 5 min at 37°C. Then 10 mL of riboflavin was added to each sample and incubated again for 8 min at 40°C. Absorbance was measured at 560 nm three times for each sample.
Percentage NBT was calculated by formula: (absorbance of blankabsorbance of sample)/(absorbance × Blank) 100. The amount of enzyme causing 50% inhibition in NBT reduction rate is defined as one unit of SOD activity. The SOD activity was measured in unit/min scale.
Catalase (CAT) assay: For CAT assay 50 mM potassium phosphate buffer (1990 mL), hydrogen peroxide (1000 mL) and homogenate sample (100 mL) were taken in a cuvette. Absorbance was measured at 240 nm three times for each sample at 30 s time intervals. Oneunit activity of catalase was described as an absorbance change of 0.01 as unit/min.
Peroxidase (POD) assay: Reaction mixture was prepared by adding 50 mM phosphate buffer (2.5 mL) of pH 5.0, 20 mM guaiacol (0.3 mL) and enzyme extract (0.1 mL). The mixture was vigorously mixed until it became homogenous solution. It was then poured into a cuvette, and finally 40 mM hydrogen peroxide was added to the reaction mixture. Change in the absorbance of reaction mixture was measured after 1 min at 470 nm. Three readings were taken at every 30 s intervals for each sample. One unit of Peroxidase activity was described as an absorbance change of 0.01 as unit/min.
Reduced Glutathione (GSH) assay: The reaction mixture was prepared by mixing tissue homogenate (0.1 mL), 0.4 M disodium hydrogen phosphate buffer (1 mL) and 5, 50-Dithio-Bis 2-Nitrobenzoic Acid (DTNB) reagent (0.5 mL). DTNB also known as Ellman’s reagent was prepared by mixing 40 mg of DTNB in 100 mL of 1% trisodium citrate. The absorbance of the yellow color developed was measured at 412 nm. Three readings for each sample were taken and averaged.
Statistics
Data are stated as mean ± SEM (Standard Error of Mean). Oneway analysis of variance (ANOVA) was applied using the graph pad prism (version 7.0 Microsoft Inc, California, USA). Graphical presentation of data was done by using the value p<0.05 was considered to be statistically significant variance level. For comparison among experimental groups post hoc Tukey’s test was used.
Signs of toxicity
No mortality occurred during experimental period. Generally the signs of toxicity observed were diarrhoea after one week, anorexia, reduction in growth, less activity, abnormal gait score and raise of body fur was observed in Sodium Metabisulphite treated group.
Relative increase in body weight
On day 56th the relative increase in body weight was measured, SMBS treated rats showed a significant decrease in body weight compared to control, in combination with vitamin and vitamin C alone (Figure 1).
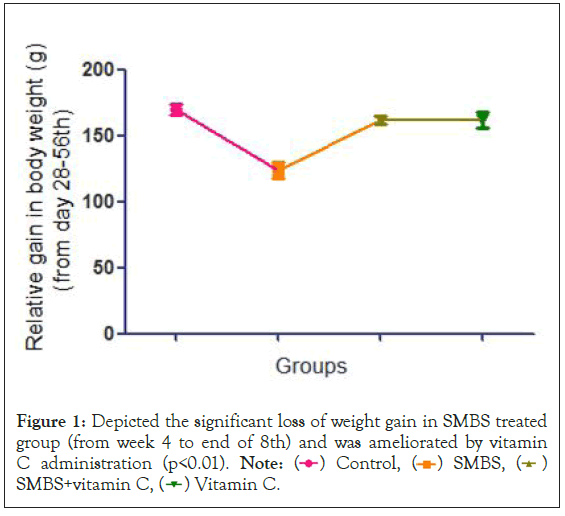
Figure 1: Depicted the significant loss of weight gain in SMBS treated
group (from week 4 to end of 8th) and was ameliorated by vitamin
C administration (p<0.01).
Hematological parameters
Complete blood count parameters were measured and compared relative to the control in Table 1. Analysis of hematological parameter revealed a reduction in hemoglobin, Red Blood Cell Count, hematocrit, Mean Crepuscular Volume (MCV), Mean Crepuscular Hemoglobin (MCH), Mean Crepuscular Hemoglobin Concentration (MCHC), RBC Distribution Width (RDW), while increase in White Blood Cells Count (TLC), absolute leukocyte count (neutrophils, eosinophils, monocytes and lymphocytes), platelet count and mean platelet volume in the sample blood.
| Parameter | Control | SMBS | SMBS+vitamin C | Vitamin C |
|---|---|---|---|---|
| Hemoglobin | 13.90 ± 0.26 | 11.90 ± 0.71a*** | 12.76 ± 0.95a* | 14.18 ± 0.82b***c** |
| RBC Count (× 106/ul) | 6.73 ± 0.18 | 5.75 ± 0.45a*** | 6.18 ± 0.41a* | 6.46 ± 0.34b*** |
| Haematocrit (%) | 42.16 ± 0.73 | 35.07 ± 2.71a*** | 39.66 ± 2.41a**b** | 42.97 ± 0.65b***c*** |
| MCV (fl) | 63.95 ± 1.38 | 61.30 ± 1.83a** | 62.02 ± 1.67 | 64.46 ± 1.11b**c* |
| MCH (pg) | 22.32 ± 0.36 | 20.00 ± 0.75a*** | 21.00 ± 0.75b*** | 22.63 ± 0.77b***c*** |
| MCHC | 33.28 ± 0.45 | 30.08 ± 0.80a*** | 32.18 ± 2.12a* | 33.16 ± 0.73b***c* |
| RDW (%) | 14.66 ± 0.86 | 16.81 ± 0.72a*** | 15.82 ± 0.50a** | 14.77 ± 1.13b***c* |
| TLC | 8019 ± 419 | 10331 ± 450a*** | 11825.0 ± 267.3a*** | 8427 ± 447b***c*** |
| Neutrophils | 39.75 ± 3.96 | 69.38 ± 11.16a*** | 51.88 ± 11.93a*b** | 40.50 ± 1.77b*** |
| Lymphocytes | 36.38 ± 4.69 | 47.88 ± 7.00 | 51.63 ± 24.14 | 37.13 ± 3.18 |
| Monocytes | 4.12 ± 1.12 | 8.00 ± 0.92a*** | 5.75 ± 1.90b** | 4.50 ± 1.06b*** |
| Eosinophils | 4.87 ± 1.24 | 9.37 ± 1.40a*** | 11.62 ± 2.26a*b** | 5.75 ± 1.03b*** |
| Platelet Count (× 103/ul) | 662.6 ± 93.9 | 948 ± 110.0a*** | 818.1 ± 144.8a* | 728.6 ± 79.8b** |
| MPV (fl) | 8.90 ± 0.65 s | 11.25 ± 0.53a*** | 9.48 ± 0.83b*** | 8.87 ± 0.48b*** |
Note: a: Significantly different from control; b: Significantly different from SMBS treated; c: Significantly different from SMBS and vitamin C combine treated, SMBS: Sodium Metabisulphite. (*p<0.05; **p <0.01; ***p<0.0001).
Table 1: Complete blood profile showing hematological parameter in Control, SMBS, SMBS+vitamin C and vitamin C alone treated groups after 25 day of daily oral exposure of experimental treatments.
Serum chemistry
Liver function test showed that the activity of ALT, AST and ALP and kidney function test urea and creatinine was increased significantly (Table 2) in Sodium Metabisulphite when compared with the control that was ameliorated by vitamin C showing that Sodium Metabisulphite can be a potential toxin for the liver and kidney. Serum oxidative stress biomarkers
| S. No | Serum parameter | Control | SMBS | SMBS+vitamin C | Vitamin C |
|---|---|---|---|---|---|
| 1 | AST (GOT) (U/L) | 51.37 ± 4.383 | 92.89 ± 12.51a*** | 66.48 ± 8.83a*b*** | 48.24 ± 5.219b***c** |
| 2 | ALT (GPT) (U/L) | 69.57 ± 7.40 | 100.9 ± 6.60a*** | 66.52 ± 6.60b*** | 53.19 ± 8.80b***c* |
| 3 | ALP (U/L) | 93.82 ± 12.56 | 152.2 ± 11.67a*** | 115.7 ± 11.11a*b*** | 96.79 ± 10.92b***c* |
| 4 | Urea (mg/dl) | 21.33 ± 3.67 | 44.03 ± 4.96a*** | 30.06 ± 3.044 | 23.09 ± 2.19b*** |
| 5 | Creatinine (mg/dl) | 0.2215 ± 0.058 | 0.4431 ± 0.130a*** | 0.3262 ± 0.0689 | 0.1969 ± 0.036b*** |
Note: Data is expressed as mean ± SD. Different superscript letters in the same row indicate statistical significance. a: Significantly different from control; b: Significantly different from SMBS treated group; c: Significantly different from SMBS+vitamin C treated; SMBS: Sodium Metabisulphite. (*p<0.05;**p <0.01;***p<0.001).
Table 2: Effect of oral administration of Sodium Metabisulphite (SMBS) and its combination with vitamin C on serum biochemical parameters.
Antioxidant profile: In serum activities of antioxidant enzymes, the SOD (Figure 2), CAT (Figure 3) and POD (Figure 4) decreased significantly (p<0.05) in Sodium Metabisulphite treated group as compared to control and other treatment groups. Levels of Reduced Glutathione, the GSH also showed significant follow the same pattern (Figure 5).
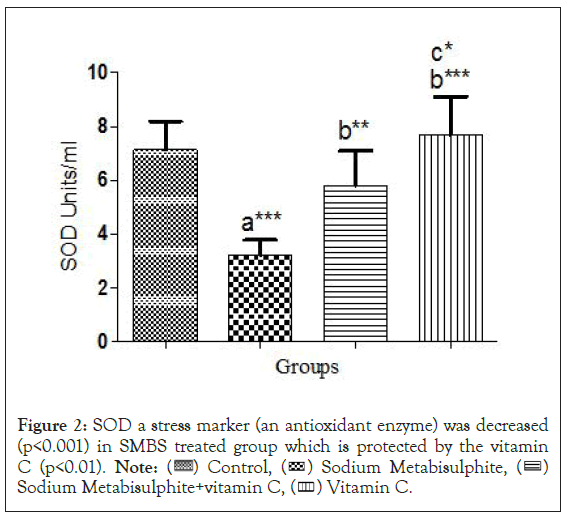
Figure 2: SOD a stress marker (an antioxidant enzyme) was decreased
(p<0.001) in SMBS treated group which is protected by the vitamin
C (p<0.01).

Figure 3: Antioxidant properties of vitamin C against SMBS induce
toxicity was significant. Comparison was showed against control
with SMBS, SMBS+vitamin C and vitamin C alone (p<0.001).
Figure 4: Peroxidase activity profile in serum was showed in SMBS
treated and in combination with vitamin C for 25 days. POD
activity was decreased significantly in comparison to that of control.
Figure 5: Protective effect of vitamin C was found to increase significantly (p<0.05), reduced glutathione whereas oxidative stress caused by SMBS
reduced it significantly (p<0.01). Reactive oxygen species level of SMBS was increased significantly (***p<0.001) while that effect of SMBS was
ameliorated by vitamin C administration, for 28 days in combination. a: Significantly different from control; b: Significantly different from SMBS;
c: Significantly different from SMBS + vitamin C; (*p< 0.05; **p<0.01; ***p<0.001).
Oxidative stress markers: Both ROS (Figure 6) and TBARS concentrations (Figure 7) increased significantly (p<0.05) in serum of Sodium Metabisulphite treated group as compared to control and all other treated groups.
Figure 6: Reactive oxygen species level of SMBS was increased significantly (***p<0.001) while that effect of SMBS was ameliorated by vitamin
C administration for 28 days in combination. Vitamin C.
Vitamin C.
Figure 7: Defensive properties vitamin C against SMBS toxicity was significant. Comparison of control with SMBS group and SMBS group with
SMBS+vitamin C and vitamin C alone was significant (p<0.001). a: Significantly different from control; b: Significantly different from SMBS; c:
Significantly different from SMBS + vitamin C; (*p< 0.05; **p<0.01; ***p<0.001).
Serum total protein estimation
Total protein level in serum was decreased significantly (p<0.05) in Sodium Metabisulphite treated group and all other treated groups compared to control (Figure 8).
Figure 8: Protein concentration level of SMBS treated group was decreased significantly (p<0.001) that was protected (p<0.05) by vitamin C. a:
Significantly different from control; b: Significantly different from SMBS; c: Significantly different from SMBS+vitamin C; (*p< 0.05; ***p<0.001).
The present study was conducted to in vivo investigation of the role of vitamin C against Sodium Metabisulphite induced toxicity in rats. It also evaluated the effect of sulphites on the growing age of rats at which they are probably more vulnerable to be affected by such kinds of toxins because of their high food intake and metabolic rate. Sulphites doses administered presently were determined according to previous studies [30]. The dose of vitamin C was administered 30 min before the administration of Sodium Metabisulphite because it has been found in previous studies that pre-treatment with antioxidant vitamins reduced the generation of ROS thus prevents the toxins induced derangement in the activities of the antioxidant enzymes and the protective effect of the pretreatment with vitamin C against imidacloprid-induced oxidative stress in liver mice is better than the post-treatment [31,32].
Hematological parameter results are in agreement with the previously conducted studies which showed the appearance of anaemia at 2% and a leucocytosis to 6% of Sodium Metabisulphite in wistar rats. According to Gunnison, et al. [33], rats fed a diet containing 6% of Sodium Metabisulphite become severely anaemic after 21 days [34]. It is already demonstrated by another study that administration of sulphites in rats provoke a significant reduction in HCT, RBC, Hb, MCHC, WBC, and platelets count were observed receiving 500 and 1000 ppm sulphites. This reduction might be due to the oxidative damage. This damage can also be attributing to lysis or possibly shrinkage of erythrocytes in blood [35]. Reduction in hemoglobin content may be attributed to reduction in RBCs concentration. The lower Hb content is due to the inadequate supply of iron for the synthesis of haemoglobin [36]. Reduction in hematocrit percentage may be attributed to the decrease in the size of RBCs and the decrease in hemoglobin synthesis, which in turn regulates the maturation of erythrocytes. The MCH concentration is in actual represents the content of hemoglobin in erythrocytes cytoplasm. Thus MCV and MCHC are directly depended upon the RBCs, whereas Hb content value followed the same trend [37]. Possible reason for the reduction in MCHC is linked with the toxicity induced by Sodium Metabisulphite in bone marrow that loose the capacity to synthesize hemoglobin at a required rate.
Sodium Metabisulphite reacts with water giving rise to sulphite, and it is efficiently absorbed in the dose administered, thereby increasing the concentration of S-sulfonate in plasma, a commonly used indicative of sulphite intake [9,34]. In similar way another study demonstrated that urinary concentration of sulphite was increased in the sulphite oxidase deficient animals receiving metabisulphite [38]. While higher activity of sulphite oxidase by rat liver prevents that accumulation so this enzyme maintains sulphite at low levels [39].
It was observed that administration of sodium sulphite to rats, the level of ALT, AST, and ALP, as well as urea, creatinine showed a significant increase. These results are highly consistent with a recently conducted study, which showed that liver and renal functional markers increased with the increase in sulphite exposure [35]. Similarly, treatment of sodium thio-sulphate provokes same enzyme changes in serum [40]. Any damage to liver results in an elevation of both ALT and AST in blood. Liver enzymes such as AST, ALT and ALP are fundamental cellular enzymes that are present in low concentrations in serum under normal conditions and determine its functionality. ALP level in serum is considered as the first sign of cell and liver damage [41]. The high level of enzymes activity like Aspartate Aminotransferase (ASAT) Alanine Aminotransferase (ALAT) and Alkaline Phosphate is linked to the hepatotoxic effect that is caused by xenobiotics. As liver is the principal organ for detoxification because it contains most of the enzymes of metabolism for the xenobiotics. In recent studies it is proposed that the relative weight of liver, kidney, spleen and stomach described a significant enlargement in size in response to sulphite exposure. Enlargement of liver and kidney is an indirect measure of functional disruption of hepatic and renal enzymes (increased level of AST, ALT, ALP, urea and creatinine) [42]. The increase in the total leukocytes counts may be due increase in spleen weight, result in increased extra-medullary splenic hematopoietic activity and thus increased leucocyte counts. These results are fully consistent with the previous studies [30], which showed the presence of splenomegaly in rats receiving doses of 4% or more of Sodium Metabisulphite in a follow-up study, they showed an enlarged liver, kidney and heart of pigs treated with 0.83% and 1.72% of Sodium Metabisulphite [14].
Rise of creatinine and urea levels may indicate a reduction in the glomerular filtration rate as a result of an acute renal dysfunction [43]. Present study showed significant difference in serum parameters such as ALT, AST, ALP, urea and creatinine and indicated that Sodium Metabisulphite affected the kidney and liver function. Similar results were reported and found that food additive such as potassium bromate induced an acute kidney dysfunction. In agreement to this present study showed that kidney tubular morphology was destroyed by sulphite exposure. Uric acid is the final product of the catabolism of purine [44]. The levels of uric acid in most mammals are lower than in humans due to the presence of uricase, a liver enzyme that degrades uric acid into allantoin [45]. The present results show hyperuricemia in sulphite treated group that was similar to the observation in previous conducted study [35]. The observed increase in serum levels of uric acid may be the result of reduced urinary excretion of the metabolite. Creatinine and urea are excellent markers of renal function, and their increase or decrease reflects a dysfunction of the renal function [46]. Transaminases are enzymes with important metabolic activity inside cells. Their increase in serum reflects cell damage, in particular at the hepatic level [47]. The results indicate that Sodium Metabisulphite cause an increase in serum urea, creatinine defining the abnormal renal and hepatic function as reported by previous studies.
In this study, the effects of Sulfite on serum stress marker profile. The present study indicated that sulphites induced significant increases in serum ROS and TBARs. These finding are similar to those observed in previous studies TBARS levels, an end product of lipid peroxidation, increased in the erythrocytes of sulphite exposed normal (CS) and deficient groups (DS) suggesting the presence of increased oxidative stress [48]. These results also confirmed previous observations of sulphite-induced lipid oxidation and demonstrated it in vivo occurrence [49]. According to present results the level of antioxidant enzymes such as Peroxidase (POD), Catalase (CAT), Superoxide Dismutase (SOD), and Reduced Glutathione (GSH) were decreased significantly in Sodium Metabisulphite treated group. One of the previous study describe similar results that sulphite treatment may cause oxidative stress and increases activity of antioxidant enzymes in all treatment groups compared to control [49]. GSH is an antioxidant, non-protein thiol, most abundant in cells and is the key non-enzymatic antioxidant defence inside brain cells. Therefore, it acts as a reactive species scavenger and a substrate for antioxidant enzymes, such as glutathione peroxidase and GST [50,51]. Another study demonstrated that sulphite decreased the activities of Glutathione Stransferase (GST) and Glutathione Reductase (GR) in cerebral cortex and of GST in cerebellum of SOdeficient rats [38]. Moreover, the decrease of GST and also of GR activity may have been also caused by sulphite-generated reactive species attacking critical amino acid residues of these enzymes structures, since previous data showed that these antioxidant enzymes can be inactivated by oxidation [52,53]. Considering that GR is critical for the reduction of GSSG to GSH and the maintenance of a reducing intracellular environment [54-56], it is presumed that the inhibition of this enzyme in cerebral cortex strongly contributes to the depletion of GSH observed. Regarding GST, this enzyme uses GSH as substrate to detoxify potentially prooxidant electrophiles, which include products of oxidative damage, such as organic peroxides. It has been verified that reduction in the supply of GSH in combination with a decreased GST activity may lead to the accumulation of oxidative damaged molecules and aggravate oxidative stress [54,56]. Under normal conditions, a delicate balance exists between rates of free radical formation and their removal by antioxidant enzymes. Although an increment of one of the antioxidant enzymes activities may have a protective role, this is only true when adequate activity of the others exists. In the present studies level of antioxidant fell down probably due to continuous production of free radical due to the oral exposure of sulphite [57].
Due to the generation of very reactive ionic species and potentially toxic interactions with molecules of biological importance, sulphite levels must be strictly regulated, via mechanisms such as Sulphite Oxidase (SOX) mediated detoxification. Studies have shown an inverse correlation exists between SOX levels in liver and sulphite toxicity in several species of laboratory animals [58]. Probably it may increase the activity of antioxidant enzymes and cause reduction in the level of these enzymes as described by the present study results.
In agreement with the present study previous studies indicated that SOD, CAT, and GPx activities were significantly increased in hippocampus with sulphite exposure in young groups. This significant increase of hippocampus antioxidant capacity in the sulphite group may be an adaptive response to sulphitedependent oxidative stress [49,59]. But when the supply of these antioxidant enzymes was not sufficient it may lead to reduction in concentration of these enzymes as observed in our studies as supported by the previous studies that reduction in Reduced Glutathione concentration, Glutathione Reductase and Glutathione S-Transferase (GST) activities in cerebral cortex and of GST in cerebellum of sulphite oxidase deficient rats were observed after oral exposure of sulphites [38]. It possibly also suggested the reduction in the motor activity, learning and memory in present studies that may be due to the less activity of neurons due to oxidative stress in brain.
According to the previous studies it has been suggested that lipid peroxidation status can be affected by sulphite exposure [60-63]. In the present study the level of TBARS increased significantly after sulphite exposure that results are highly consistent with the previous study, following sulphur dioxide exposure, there was an increase in TBARS production in all age-groups; however, the extent of this increase was greater in young rats compared to middle- and old-aged rats .These studies are linked with the present study and suggested that at early stages of life young ones are probably more vulnerable to sulphite toxicity than other [64]. Therefore, from the results of this study as well as in previous study, suggested that the lipid per oxidative effect of sulphite might be masked by increased lipid peroxidation with age. According to the recent technical report policy statement by American Academic of Paediatrics, that suggested food additive are putting children health at risk. The technical report identifies bisphenols, phthalates and nitrates or nitrites as additives with evidence of concern (to be made).
The present study demonstrated that administration of vitamin C with Sodium Metabisulphite significantly reduce the oxidative stress in experimental rats. This can be elaborated by antioxidant ability of vitamin C as demonstrated in previous studies that primary role of vitamin C is to neutralize free radicals; it can work both inside and outside the cells. Free radical will seek out an electron to regain their stability, vitamin C is an excellent source of electrons; therefore, it can donate electrons to free radicals such as hydroxyl and superoxide radicals and quench their reactivity [65]. Vitamin C is thought to be an important water soluble antioxidant which is reported to neutralize ROS and reduce the oxidative stress [66-69]. Pre-treatment with antioxidant vitamins decreased the generation of ROS thus prevented the pesticide induced derangement in the activities of the antioxidant enzymes [70]. Similarly, vitamin C, a water soluble vitamin, is effective in scavenging free radicals, including hydroxyl radicals, aqueous peroxyl radicals and superoxide anions. Ascorbic acid act as two electron reducing agent and confers protection by contributing an electron to reduce free radicals, thus neutralizing these compounds in the extra- cellular aqueous environment prior to the reaction with biological molecules [27,28]. Moreover, the antioxidant potential of ascorbic acid is not only attributed to its ability to quench reactive oxygen species, but also to its ability to regenerate others mall antioxidant molecules, such as tocopherol, glutathione and carotene.
Conclusively the present study investigated the role of vitamin C against Sodium Metabisulphite induced toxicity in pre-pubertal rats in vivo. Sub acute exposure to SMBS induces wide range of pathophysiological hallmarks accompanied by hematological, biochemical, and antioxidants variations in neonatal rats. We investigated potential hazardous effect of sulphites on the growing age of rat at which they are probably more vulnerable to be affected by such kinds of toxins because of their high food intake as well as metabolic rate.
I affirm that the research work is outcome of my own effort, and that I composed article myself. No part of this research has been published in any journal.
The author is thankful to graduate school of health sciences, KOC University, Istanbul, Turkey and animal research ethics committee for providing rats for experimental purpose.
This study was approved from ethical committee of KOC University, graduate school of health sciences, Istanbul, Turkey. All of animal procedures were carried out under the standard rules established by bioethical committee of university. The author declared consent to participate.
The author declared no conflict of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Aslam M (2022) Ascorbic Acid Mitigates the Sodium Metabisulphite Induced Neonatal Pathophysiology: Study Conducted in Rodents. J Clin Toxicol. 12:508.
Received: 04-Apr-2022, Manuscript No. JCT-22-16547; Editor assigned: 06-Apr-2022, Pre QC No. JCT-22-16547; Reviewed: 20-Apr-2022, QC No. JCT-22-16547; Revised: 27-Apr-2022, Manuscript No. JCT-22-16547; Published: 04-May-2022 , DOI: J Clin Toxicol, Vol.12 Iss.2 No:1000508
Copyright: © 2022 Aslam M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.