Journal of Horticulture
Open Access
ISSN: 2376-0354
ISSN: 2376-0354
Research Article - (2015) Volume 2, Issue 4
The study focused to evaluate cytotoxic, antioxidant, antimicrobial and anticholinesterase activities of methanol extracts of Pleurotus ostreatus Jacq. (Pleurotaceae), Boletus edulis Bull. (Boletaceae), Tricholoma populinum J. (Tricholomataceae) Helvella queletii Bres. (Helvellaceae), Armillaria tabescens Emel. (Physalacriaceae), Psathyrella candolleana Fr. (Psathyrellaceae) and Helvella leucopus Pers. (Helvellaceae) mushroom species. Phenolic acid profiles of these mushrooms were also determined to obtain further information on the correlation between the contents of phenolic compounds and studied activities. Cytotoxic activity of mushrooms was screened by MTT cytotoxicity assay on cancer (HeLa) and normal epithelium (NRK-52E) cell lines. To determine antioxidant potential of mushroom extracts free radical scavenging, reducing power, superoxide anion radical scavenging, total antioxidant and metal chelating activities were studied, To indicate anthicholinesterase activity the acetyl-and butyrylcholinesterase inhibitory activities of the mushroom extracts were studied. For antimicrobial activity disc diffusion method was applied. Phenolic profile of mushrooms were determined by HPLC system. The IC50 values of the extracts were 1.58-25.11 and 2.05-22.32 mg/mL for HeLa and NRK-52E cells, respectively. At antimicrobial activity the inhibiton zones were found to be as 1 ± 0.12-13 ± 0.23 mm. P. ostreatus, B. edulis and H. leucopus extracts were showed higher activities than the other mushroms at antioxidant, antimicrobial, anticholinesterase and cytotoxic activity.
<Keywords: Cytotoxic activity; Antioxidant activity; Anticholinesterase activity; Antimicrobial activity; Phenolic acids; Mushroom
Natural products, including phenolic compounds, polyketides, terpenes and steroids synthesized by mushrooms have been attractive for their medicinal properties like antioxidant, antimicrobial, antitumor and cytotoxic etc. [1]. Numerous molecules isolated from mushrooms are known to be bioactive, including polysaccharides, glycoproteins, terpenoids, β-glucans and lectins [2]. A wide variety of naturally occurring substances have been shown to protect against tumor development [3] and inflammatory processes [4]. Recent scientific evaluations of macrofungi, such as mushrooms and entomopathogenic fungi, have confirmed the efficacy of extracts from either the fruiting bodies or mycelia of these species in inhibition of the proliferation of various cancer cells lines [5,6]. Antioxidants have been widely used as food additives to provide protection against oxidative degradation of foods [7]. Oxidation of lipids, which is the main cause of quality deterioration in many food systems, may lead to off-flavors and formation of toxic compounds, and may lower the quality and nutritional value of foods. Furthermore, lipid oxidation is also associated with aging, membrane damage, heart disease and cancer [8]. At the present time, the most commonly standard antioxidants are Butylated Hydroxy Anisole (BHA), Butylated Hydroxy Toluene (BHT), propyl gallate (PG) and tert butylhydroquinone (TBHQ). The safety of these antioxidants has recently been questioned due to toxicity [9]. Therefore, there is a growing interest on natural and safer molecules for different applications. For this purpose many scientist have studied bioactive potential of different molecules from mushrooms [10-16]. In present experimental study we focused to evaluate cytotoxic, antioxidant, antimicrobial and anticholinesterase activities of methanol extracts of Pleurotus ostreatus, Boletus edulis, Tricholoma populinum, Helvella queletii, Armillaria tabescens, Psathyrella candolleana and Helvella leucopus mushroom species. Beside these studies phenolic acid profiles of mentioned mushrooms were also determined to obtain further information on the correlation between the contents of phenolic compounds and studied activities.
Chemicals
Polyoxyethylenesorbitan monolaurate (Tween-20), 1,1-diphenyl-2- picryl-hydrazyl (DPPH), ferrous chloride, α-tocopherol, 3-(2- pyridyl)-5,6-bis (4-phenyl-sulfonic acid)-1,2,4-triazine (Ferrozine), butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) ascorbic acid, trichloracetic acid (TCA), acetic acid, nitro blue tetrazolium (NBT), acetone, linoleic acid, galanthamine hydrobromide and trolox were purchased from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany), acetylcholinesterase, butyrylcholinesterase, from Sigma (Germany) acetylthiocholine iodide from Aldrich (Germany), butyrylthiocholine iodide from Fluka (Germany), sodium dihydrogen phosphate, sodium hydrogen phosphate, from Reidel de Haen (Germany) Ammonium thiocyanate, methanol was purchased from E. Merck. Blank antimicrobial susceptibity test discs were purchased from Oxoid. All other chemicals were analytical grade and obtained from either Sigma-Aldrich or Merck.
Mushrooms
The mushrooms studied were collected from Eruh-Siirt region located the south-east region of Turkey during March, April and May of 2011-2012.
Preparation of the methanol extracts of mushrooms
The fruiting bodies of mushrooms were divided into small pieces were air-dried in an oven at 40°C. Dried mushroom samples (10 g) were powdered in a blender and then extracted by stirring with 100 ml of methanol at 30°C at 150 rpm for 24 h and filtered through Whatman No. 4 filter paper. Additionally two 100 ml portions of methanol were used for re-extracting the residue as described above. The combined methanol extracts were then evaporated at 40°C and extraction yields were calculated for each mushroom. For preparing stock solution (25 mg/ml) crude extracts were redissolved in methanol and stored at 4°C for further use.
Determination of phenolic compounds of mushrooms
Sample preparation: Each mushroom sample (~3 g) was extracted using 30 ml acetone-water mixture (80:20) at -20°C during 6 h. After treated for 15 min. in an ultrasonic bath, the extracts were centrifuged at 4000 g for 10 min (5430 R model, Eppendorf), and filtered through Whatman no. 4 paper. For extraction of the residue, two additional 30 ml portions of the acetone: water mixture were used. The combined extracts were evaporated to remove acetone at 40°C by a nitrogen evaporator (TAB-40 WEL model, Teknosem). The aqueous phase was washed with n-hexane and then submitted to a liquid-liquid extraction with diethyl ether (3 × 30 ml) and ethyl acetate (3 × 30 ml). At 40°C the organic phases were evaporated and redissolved in water-methanol (80:20). Finally, prepared solutions were filtered through a 0.22 μm disposable LC filter disk for HPLC analysis.
HPLC analysis: The phenolic extracts of mushroom species were determined by using HPLC equipment consisting of an integrated system with an Agillent 1260 Infinity HPLC-DAD. Data were analysed using Agilent Chem Station revision B.04.01 software (Agilent). The chromatographic separation was achieved with an Agillent ZORBAX reverse phase C18 column (250 × 4,6- 5 μm) thermostatted at 35°C. For gradient elution, two solvents were used: One of them consists of acetic acid–water (2:98 v/v) and the other was consists of only methanol. Injection volume was 20 μl. Detection was carried out in a DAD, using 280 nm as the preferred wavelength.
Determination of antimicrobial activity of mushroom extracts
Test microorganisms: Escherichia coli (ATCC 10536), Staphylococcus aureus (ATCC 6538), Bacillus subtilis (6051), Enterococcus hirae (ATCC 10541), Micrococcus luteus (ATCC 9341), and Pseudomonas aeruginosa (ATCC 9027) were used as the test microorganisms. Nutrient broth (NB) was used for culturing of test bacteria. All strains were regenerated twice before use in the antimicrobial test.
Disc diffusion method
Disc diffusion method [17] was used for determination of antibacterial activity of P. ostreatus, B. edulis, T. populinum, H. queletii, A. tabescens, P. candolleana and H. leucopus methanolic extracts on test bacteria. Bacterial cultures were incubated at 37°C for 24 h in Nutrient Broth. 25 ml of Nutrient Agar were poured into petri dishes. 100 μl of the bacteria culture suspension were inoculated to petri dishes and dealt with a drigalski spatula. The blank discs (7.0 mm, Oxoid Ltd, Wade Road, Basingstoke, Hants, RG24 8PW, UK.) were impregnated with 100 μl extracts (10 mg/ml concentration), the same volume (100 μl) of methanol was used as a control. The inoculated plates were incubated for 24 h. After incubation, the diameter of the inhibition zone was measured with calipers. The measurements were done basically from the edge of the inhibition zone to the edge of the discs.
Determination of antioxidant activity of mushroom extracts
Free radical scavenging ability: In this test, Blois (1958) method was used for determining DPPH scavenging ability by a spectrophotometric method based on the reduction of a methanol solution of DPPH. Each extract (1, 2, 5, 10, 15 and 20 mg/ml) in methanol (1 ml) was mixed with 4 ml of methanol solution containing DPPH radical. The mixture was shaken vigorously and left to stand for 30 min in the dark and the absorbance was then measured at 517 nm against a blank (methanol). Inhibition of free radical, DPPH, in percent (I%) was calculated according to the formula:
where Acontrol is the absorbance of the control reaction (containing all reagents except for the mushroom extracts), and Asample is the absorbance of the test compound. Tests were carried out in triplicate. BHA and BHT were used as positive control.
Reducing power
The reducing power of methanolic extracts of mushroom species was determined according to the method of Oyaizu (1986). Each extract (1, 2.5, 5, 7.5 and 10 mg/ml) in methanol (2.5 ml) was mixed with 2.5 ml of 200 mM sodium phosphate buffer (pH 6.6) and 2.5 ml of 1% potassium ferricyanide and the mixture was incubated at 50°C for 20 min. Then, 2.5 ml of 10% trichloroacetic acid were added, and the mixture was centrifuged at 200 rpm for 10 min. 2.5 ml of deionized water and 0.5 ml of 0.1% ferric chloride were mixed with the upper layer (2.5 ml) of the solution. Finally the absorbance was measured at 700 nm against a blank. Trolox and ascorbic acid were used as control [18,19].
Metal chelating ability
The chelating ability of extracts was determined according to the method of Dinis et al. [20]. Each extract (0.1–4 mg/ml) in methanol (1 ml) was added to 3.7 ml of methanol and mixed with 0.1 ml of 2 mmol/l ferrous chloride. The reaction was started by adding 0.2 ml of 5 mmol/l ferrozine. The mixture was left for standing 10 min at room temperature, then the absorbance was measured at 562 nm against blank. The results were expressed as percentage of inhibition of the ferrozine -Fe2+ complex formation. EDTA was used as a positive control. The percentage inhibition of the ferrozine -Fe2+ complex formation was calculated using the formula given below:
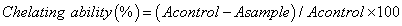
Superoxide anion radical scavenging activity
The method of Zhishen et al. [21] was performed. All solutions were added to 0.05 M phosphate buffer (pH 7.8). To start the anion radical formation in reactants by photo-induced reactions, 4000 1 × of illumination intensity was adjusted in a closed box with fluorescent lamps. The total volume of reactant was 5 ml and the concentration of riboflavin, methionine and NBT were 4.0, 2.0 and 3.0 M respectively. The reactant was illuminated at 25°C for 25 min. The photochemically reduced riboflavins generated anion radicals, which reduced NBT to form blue formazan. The un-illuminated reaction mixture was used as a blank. Absorbance was measured at 560 nm. Methanolic extracts of mushroom species and standards were added to the reaction mixture, in which anion radical was scavenged, thereby inhibiting the NBT reduction. Absorbance (A1) was measured and decrease in anion radical was represented by A–A1. The degree of scavenging was calculated by the following equation:

Total antioxidant activity by the ferric thiocyanate method
Ferric thiocyanate method [22] was used for settling the antioxidant capacity of mushroom extracts. A volume of 100 μl (0.1 mg/mL) of each extracts was mixed with 0.05 M phosphate buffer at pH 7.4 and 0.07 M linoleic acid was solved in tween 20 to obtain 4 ml of solution. The final solutions were incubated at 37°C in prosody caps. 100 μl of aliquot from each extract were removed periodically, and added to FeCl2-ammonium thiocyanate solution. During the linoleic acid oxidation, peroxides formed which oxidized Fe+2 to Fe+3. The Fe+3 ions form a complex with SCN¯, and this complex has a maximum absorbance at 500 nm. This step was repeated in every 24 h until the control (phosphate buffer, linoleic acid mixture) reached its maximum absorbance value. Therefore, high absorbance values indicated high levels of linoleic acid oxidation. Phosphate buffer was used as the reaction blank. The total antioxidant activity was expressed as the average of three independent determinations carried out in duplicate. The percentage inhibition of lipid peroxidation of linoleic acid was calculated by applying the following equation: inhibition of lipid peroxidation
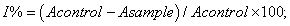
where Acontrol is the absorbance of the control reaction (phosphate buffer plus linoleic acid), and Asample is the absorbance obtained in the presence of the extracts or positive control of antioxidant activity (BHA and BHT).
Anticholinesterase activity
A spectrophotometric method developed by Ellman et al. [23] was established to indicate the acetyl- and butyryl-cholinesterase inhibitory effects.
Cytotoxic activity
Cell lines, culture treatments: The cytotoxic effect of mushroom extracts were assessed by using MTT test on human cervix cancer (HeLa) and rat kidney epithelium cell (NRK-52E) lines. NRK-52E (ATCC CRL-1571) and HeLa (ATCC CCL-2) were cultured according to manufacturer’s protocols. Following steps were carried out for both cells. Cells were seeded at 104 cells/100 μl into each well of 96-well plates. After 24 h of incubating period, culture medium was removed. Then, the extracts were added to wells in various concentrations. The exposure of concentrations were determined as μg/ml for the extracts. After 24 h of incubation with the extracts, MTT cytotoxicity test was performed to assess the viability of normal and tumor cells exposed to mushroom extracts.
MTT cytotoxicity test
The test principle is that MTT, formed 3-[4,5-dimethylthiazol-2- yl]-2,5-diphenyl-tetrazolium bromide and yellow coloured water soluble tetrazolium salt, is reduced to an insoluble purple formazan product by the mitochondrial succinate dehydrogenase, which belongs to the mitochondrial respiratory chain and is only active in viable cells, in the presence of an electron coupling reagent. The protocol was performed according to the method of Alley et al. [24]. Absorbance was read at 590 nm by using microplate spectrophotometer system (BioTek® Epoch Microplate Spectrophotometer, Winooski-USA). In every test, negative (untreated, culture medium) and solvent (1% DMSO) controls were used. For each extract, four concentrations were tested in triplicates and each test was repeated twice. 50% inhibition concentration (IC50) was used for cytotoxic activities. IC50 value was expressed as the concentration of sample caused an inhibition of 50% in enzyme activities in cells. In calculation, the absorbance values of samples were compared with the absorbance values of solvent controls after all absorbance values were corrected by subtracting the absorbance of blank. In MTT test, a dose-respose curves was constructed and IC50 calculated according to the below formula as the percentages of solvent controls;
% inhibition=100-(corrected mean Asample × 100/corrected mean Asolvent control)
IC50 values are defined as the concentrations of test compounds required to reduce the absorbance to 50% of the control values.
Phenolic profile of mushrooms
In this study the phenolic compounds of mushroom species such as; protocatechuic, syringic, caffeic, vanillic, o-coumaric, p-coumaric, catechin, quercetin and rutin were determined by HPLC technic (Table I). P. ostreatus contains the analyzed phenol compounds at the highest level (1068.92 mg/kg) and this is followed by B. edulis (723.64 mg/kg), H. leucopus (523.38 mg/kg) and T. populinum (357.74 mg/kg). None of the mushrooms contains all the phenolic compounds analyzed. P. ostreatus contains o-coumaric acid (567.98 mg/kg) high level when compared with other mushrooms and it contains only five phenolic acid that analyzed.
Antimicrobial activity of extracts
For determining the antibacterial effects of P. ostreatus, B. edulis, T. populinum, H. queletii, A. tabescens, P. candolleana and H. leucopus methanol extracts were tested against E. coli S. aureus, B. subtilis, E. hirae, M. luteus and Pseudomonas aeruginosa . 1 ± 0.12–13 ± 0.23 mm. inhibition zones were obtained from the extracts as shown in Table II. When the highest antimicrobial activity was shown by P. ostreatus against P. aeruginosa (13 ± 0.23) and the weakest inhibition zone was shown by P. candolleana (1 ± 0.12 mm) extract against S. aureus . None of the extracts can showed antimicrobial activity against all of the studied bacteria. While H. leucopus could exhibited antimicrobial activity against five species of microorganisms the others were effect four species of studied microorganisms.
Antioxidant activity of mushroom methanol extracts
DPPH radical scavenging activity: The decrease in absorbance at 517 nm indicates higher free radical scavenging activity. This situation take form by the reaction between antioxidants and the radicals, thus resulting in the scavenging of the radical by hydrogen donation. The results obtained from studied highest concentration (20 mg/mL) were shown in Table III. P. ostreatus , B. edulis and H. leucopus were displayed excellent activities. All the extracts increased their antioxidant activities when the concentration increased. BHT and BHA were used for positive control. BHT demonstrated excellent activity even at the concentration of 1 ml (86.91%) but at 20 mg/ml concentration BHA (93.47%) showed a little higher activity than BHT (90.05%). As shown in Table III mushroom methanol extracts showed higher activity than standards.
Determination of reducing power
The reducing power of methanolic extracts of mushrooms at 10 mg/mL concentration were shown in Table III. Reducing power of extracts increased as the concentration increased (1-10 mg/ml). Among the mushroom methanol extracts, the highest reducing power activity was obtained from P. ostreatus as 2.52 at 10 mg/ml. Trolox and ascorbic acid positive controls showed quite higher activities than extracts.
Ferrous ion chelating activity: The chelating effect of the mushroom methanol extracts on ferrous ions increased with increasing concentration as shown in Table III. H. leucopus exhibited highest chelating activity (84.69%) and this was closely followed by P. ostreatus (83.64). EDTA was used for positive control and it showed excellent activity even at low concentrations. The activities of EDTA at 0.1 mg/ml and 4.0 mg/ml were 92.44% and 94.18% respectively.
Scavenging effect on superoxide anion radicals
The superoxide anion radical scavenging activity of mushroom methanol extracts and standard antioxidants increased with increasing concentrations (0.1, 0.25, 0.50, 1.0 and 2.0 mg/ml). In this test H. queletii showed the highest activity as 76.19% (Table III).
Total antioxidant activity
The absorbance of methanolic extracts of mushrooms was measured at 24th, 48th and 72th hr periods. From 24th to 48th period the antioxidant activities of extracts reached their maximum level. After the 48th hr activities of extracts decreased gradually, at 72th hr activities remained at their minimum level. At 48th hr, the highest activities were 75.3% for P. ostreatus, 72.34% for H. leucopus, and 68.92% for B. edulis when compared with other studied mushrooms.
Anticholinesterase activity of extracts
None of the methanol extracts of the mushrooms possessed anticholinesterase effect, only the methanol extract of P. ostreatus indicated moderate butyrylcholinesterase inhibitory activity (48.21% inhibition), this is followed by B. edulis as 36.60% and H. leucopus as 32.48% inhibitory activity at 200 μg/mL (Table IV). Galantamine was used as positive control which is used for Alzheimer's disease.
Cytotoxic activity of extracts
In the present study, HeLa and NRK-52E cell lines were used because they are most suitable and common to perform cytotoxicity studies. As can be seen in the Table 5, H. queletii, A. tabescens and P. candolleana do not show cytotoxic to any of the concentrations tested. However, B. edulis, T. populinum, P. ostreatus and H. leucopus were disturbed on mitochondrial activity of cell. The extracts were not shown selectivity to the cancer and normal cell line. The highest cytotoxic activity was exhibited by B. edulis with 1.58 and 2.05 mg/ml of IC50 values for HeLa and NRK-52E cells, respectively.
Phenolic compounds such as flavonoids, phenolic acids, and tannins are considered to play an important role in antioxidant capacity of organisms. These antioxidants have different biological activities as anti-inflammatory, anti-atherosclerotic and anticarcinogenic. Palacios et al. [25] have recently reported the phenolic compounds of B. edulis from Spain. Protocatechuic, catechin, caffeic and p-coumaric content of the study differed from our findings. Anticancer, antiallergy and antioxidant activities of catechin were reported by of B. edulis displayed stronger activity against S. aureus than our study results. Phenolic compounds are related to many biologic functions including antimicrobial and antioxidant activity [26-28]. From this point of view, for this study it could be said the cause of showing antimicrobial activity is phenolic content of the mushrooms. Sarikurkcu et al. [29] studied methanol extracts of B. edulis at DPPH scavenging activity and obtained 94.66% activity at 1.5 mg/ml concentration. Tsai et al. [30] had found almost same results at free radical scavenging activity like ours. These results explicate that methanolic extracts of mushroom species can strongly reduce the DPPH radical which contains antioxidant components that could react rapidly with DPPH radicals. Sarikurkcu et al. [29] examined B. edulis methanol extracts at reducing power activity and found 1.34 activity at the concentration of 6 mg/ml. 90.20% chelating activity was obtained at 0.5 mg/ml concentration from B. edulis methanol extracts by Sarikurkcu et al. (2008). At reducing power activity Tsai et al. [30] were found moderate activity (37.4-61.8%) at 5-20 mg/ml concentration range by using B. edulis ethanol extracts. No studies were detected about the anticholinesterase activity of the mushroom species we studied. Anticholinesterase activity of different mushroom species execute better activity than ours by [31]. In the literature, there was no study for cytotoxic activities of the mushroom species. Only, similar to our results, Tong et al. [32] have reported that the growth of Hela cells could be inhibited by P. ostreatus extracts at a concentration as low as 50 μg/ml.
Using natural foods and products in diet that display bioactivities can protect the organisms against to some illness and thus they reduces the need for synthetic drugs medicines or products which mediated damage of biomolecules. In this study, P. ostreatus, B. edulis and H. leucopus methanol extracts were showed higher activities than the other mushroms at antioxidant, antimicrobial, anticholinesterase and cytotoxic activity. This event might be concluded from higher phenolic compounds of mentioned mushrooms. Phenolics are bioactive components mentioned in many literatures. [33-35]. Not only the phenolic copounds were responsible from these activities but also polysaccaridhes, glicoproteins, peptides, β-glucans etc. are posesses many biological activities. To understand deeply which compound is spesificially can display these bioactivities in each studied test, structural definition of the compounds must be brighten. Further studies considered to be done for this purpose.
This study financially supported by Scientific Research Unit of Siirt University, Project no. 2011-SIUFED-F5.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.