
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Research Article - (2016) Volume 5, Issue 1
Anchomanes difformis (Blume) Engl. and Colocasia esculentus (L.) Schott. of the family Araceae are plants widely distributed in Africa. The leaves and roots of these plants are traditionally used to treat various disease conditions including dysentery, cough, kidney pains and stomach disorders. This study aimed at investigating the antimicrobial, antioxidant and anti-inflammatory properties of methanol extracts of A. difformis leaves (ADL) and roots (ADR), and C. esculentus leaves (CEL). Antimicrobial activity was evaluated using micro-dilution methods against typed strains of Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and clinical stains of Streptococcus pyogenes and Candida albicans. The antioxidant activities of the extracts were determined using 1, 1-diphenyl-2-picryl-hydrazyl (DPPH) free radical scavenging, total antioxidant capacity and total phenol content methods. The anti-inflammatory activity of ADL, ADR and CEL were evaluated using the carrageenan-induced foot pad oedema in 7-day old chicks. ADL, ADR and CEL demonstrated broad spectrum antimicrobial activity with MIC ranging from 12.5 to 50 mg/mL. All the extracts exhibited antioxidant activity with CEL demonstrating the highest with IC50 value of 146.9 μg/mL. The methanol extracts further demonstrated a significant anti-inflammatory (p < 0.001) at the concentrations tested with 30 and 300 mg/kg body weight each extract showing better activity than the 100 mg/kg body weight. Phytochemical screening revealed the presence of saponins, phenols, tannins flavonoids, and triterpenoids in especially in ADR and CEL. The above findings may justify the medicinal uses of the plants.
<Keywords: Colocassia esculentus; Anchomanes difformis; Antimicrobial; Inflammation; Anti-inflammatory; Antioxidant
The use of medicinal plants to treat various ailments inflicting man has been in existence since the onset of time. In Africa, about 80% of the populace relies on medicinal plants in managing various forms of infections which are endemic in the sub-region [1]. Over the years, medicinal plants have proven to be a remarkable source of newer and potent therapeutic agents and have therefore taken the central stage in most research centers in the world [2,3]. However, with the identification and development of newer and potent antimicrobial interventions to treat microbial infections, the number of resistant microorganisms being identified currently, increases exponentially. It has therefore become imperative that more medicinal plants be screened to identify and develop newer and cost-effective antimicrobial agents to treat infections caused by resistant microorganisms.
Anchomanes difformis (Blume) Engl. belongs to the family Araceae. A. difformis is a large herbaceous plant which grows in the tropical zones especially in various parts of Africa [4]. Traditionally A. difformis is used to manage a vast range of ailments in West and Central Africa. In Nigeria, a decoction of the root is used to treat cough, diabetes, dysentery and throat related problems [5]. The rhizomes are used topically as vesicants and rubefacient. Both roots and leaves are used to treat oedemas, kidney pains, jaundice and as a diuretic in treating urethral discharge [6]. Studies conducted have shown that the plant possesses insecticidal activity [7]. Methanol extract of the rhizome has also found to possess trypanocidal activity [8]. Bero et al. [9] have also shown that the plant exerts antiplasmodial activity.
Colocasia esculentus (L.) Schotts (Family Araceae) is an herbaceous perennial plant that is thought to be a native of India but is widely cultivated in the tropical Africa. Traditionally, a decoction of the leaves is drunk to promote menstruation and together with other parts of the plants, it is used to relieve stomach problems and to treat cysts. C. esculenta has been reported to possess hypoglycemic effect due to the presence of cyanoglucoside [10]. Hypolipidemic activity has also been revealed and attributed to the presence of arabinogalactan [11] and mono and digalactocyl diacylglycerols [12]. It has also been reported to possess antifungal activity due to presence of cystatin [13].
This study therefore seeks to determine pharmacological properties including antimicrobial, antioxidant and anti-inflammatory properties of the methanol extracts of A. difformis leaves (ADL) and roots (ADR) and C. esculentus leaves (CEL).
Chemicals and reagents
All chemicals and reagents were purchased from Sigma-Aldrich, St. Louis, AM, USA unless otherwise stated.
Preparation of plant materials
The leaves and roots of A. difformis and leaves of C. esculentus were collected in the month of October, 2014. The plants parts were authenticated by Dr. G.H. Sam and voucher specimens of each plant material have been deposited at the Herbarium of Department of Pharmacogncosy, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. The plants parts were washed under tap water to remove debris. The leaves samples were dried in a hot air oven at 40ºC for 48 h whiles the roots of A. difformis were cut into smaller pieces and oven-dried under similar conditions. The dried plant samples were then pulverized into coarse powder using a laboratory mill machine (Christy and Norris, London, UK).
Extraction of plant materials
A quantity of 500 g of the powdered leaves of A. difformis was soaked overnight in 2.5 L 70% v/v methanol and homogenized using ultra-turrax T-50 (Janke & Kunkel KG, Hamburg, Germany) for 3 min under ice cooling. The suspension obtained was then filtered using filter paper (Whatmann No. 10) with the aid of a vacuum pump. The residue was homogenized with more solvent and filtered to ensure maximum extraction of plant material. The filtrates obtained were then concentrated using a rotary evaporator (Buchi, Konstanz, Germany) at 40°C and the concentrates lyophilized. The dry extracts obtained were then kept in a refrigerator at 4°C until needed. The above extraction procedure was repeated for 500 g of powdered leaves of C. esculentus and 400 g of powdered root of A. difformis using 3 L and 2.5 L of 70% v/v methanol, respectively.
Phytochemical screening
Preliminary phytochemical screening was performed on all the extracts for the presence tannins, saponins, flavonoids, steroids and alkaloids [14,15].
Test organisms
The test organisms used for the antimicrobial determination included: Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 25922 and clinical strains of Streptococcus pyogenes and Candida albicans. The inoculum size of 1.0 × 106 (CFU)/mL of the test organisms was used in all the antimicrobial determinations.
Determination of antimicrobial activity
Determination of minimum inhibitory concentrations (MIC): The MICs of the extracts were determined using the micro-dilution method [3,16]. Ciprofloxacin and ketoconazole were used as standard antibacterial and antifungal agents, respectively. Stock solutions of extracts and standards were prepared. The microtitre plates were initially filled with 100 μL double strength nutrient broth (Oxoid, London, UK) and 20 μL of 24 h organisms culture suspension. Calculated volumes of the stock solutions (plant extracts, ciprofloxacin and ketoconazole) were filled into labelled wells to obtain a final volume of 200 μL with varying sample concentrations. The plates were then incubated at 37ºC for 24 h. The MIC was determined as the lowest concentration of test sample that inhibited microbial growth which was indicated by the absence of purple colouration upon the addition of 30 μL of 125 mg/ mL of 3-(4,5-dimethylthiazol -2-yl)-2,5-diphenyltetrazolium bromide) (MTT) solution [3]. The experiment was independently carried out in triplicates.
Determination of antioxidant activity
DPPH free radical scavenging activity: Antioxidant activities of the extracts were determined according to the method described by Chizzola et al. [17] using the free radical 1,1-diphenyl-2-picryl-hydrazyl (DPPH). Solutions of concentrations within the range of 7.8125 to 1000 μg/mL of the extracts and reference antioxidant (α-tocopherol) were prepared in methanol. The solutions were placed in a 96-well micro-titer plate. A concentration of 0.10 mM DPPH solution was also prepared in methanol. A volume of 10 μL of the DPPH solution was added to 100 μL of the various extracts and α-tocopherol solutions in the 96-well microtiter plates. The tubes were kept in the dark for 30 min after which absorbance of excess DPPH was measured at 517 nm using a MTP reader (MTX Lab Systems, Inc., Virginia, USA). The percentage inhibition of radical scavenging was then calculated using the following equation; Inhibition 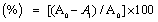 , where (A0) is the absorbance of a blank solution containing equal volume of methanol and DPPH, (A1) is the absorbance of the samples at 517 nm. Inhibitory Concentration, IC50 was determined as the concentration of sample that scavenged 50% of DPPH free radical in solution.
, where (A0) is the absorbance of a blank solution containing equal volume of methanol and DPPH, (A1) is the absorbance of the samples at 517 nm. Inhibitory Concentration, IC50 was determined as the concentration of sample that scavenged 50% of DPPH free radical in solution.
Determination of total phenolic content
The total phenolic content of the extracts was determined by the Folin-Ciocalteu method [18,19]. Hundred microliters (0.1 mL) of 0.5 N Folin-Ciocalteu reagent was added to 0.5 mL sample solutions of concentrations 1000 to 5000 μg/mL and incubated at room temperature for 15 min. Two (2) mL of 2% sodium carbonate was added to each test tube containing the extract and Folin-Ciocalteu mixture. Tannic acid of concentrations 15.6 to 125 μg/mL was used as reference substance. Absorbance was read at 760 nm with a plate reader. The experiment was independently performed in triplicates.
Determination of total antioxidant capacity
Extract concentrations ranging from 2.0 to 5.0 mg/mL were prepared. To a volume of 1 mL of the extract solutions, 3 mL of mixed reagents (28 mM disodium Phosphate, 4 mM ammonium Molybdate and 0.6 M H2SO4) solution was added and incubated at 95ºC for 90 min. Absorbance was then read at 695 nm after incubation [19].
Determination of anti-inflammatory activity
Ethical clearance: The in vivo anti-inflammatory studies were approved by the Faculty of Pharmacy Animal Ethical Committee (FPPSAEC/ CA01/13), Faculty of Pharmacy of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana and also in compliance with internationally accepted principles for laboratory animal use and care (EEC Directive of 1986: 86/609 EEC). The procedure was performed in accordance with the guide for care and use of laboratory animals.
Carrageenan-induced foot oedema: Carrageenan induced inflammation of the footpad of chicks was employed to assess the antiinflammatory property [20] of ADL, ADR and CEL. The chicks were randomly divided into eleven (11) groups with each group consisting of five (5) chicks. The chicks were weighed and their foot volumes measured using electronic calipers.
Chicks in the groups 1 to 3 were orally administered with 30, 100 and 300 mg/kg body weight of ADL, respectively. Group 4 to 6 received 30, 100 and 300 mg/kg of ADR orally, respectively. Groups 7 to 9 were orally administered with doses of 30, 100 and 300 mg/kg body weight of CEL, respectively whiles Groups 10 and 11 were administered orally with aspirin (positive control) (100 mg/kg) orally and vehicle (distilled water). Inflammation or oedema was induced by a sub-plantar injection of carrageenan (0.10 mL of a 2% w/v solution in normal saline) into the right footpad of the chicks 1 h post treatment.The foot pad volumes were determined immediately before the experiment (time zero) and every hour until 6 h post-carrageenan injection. Drug effects were evaluated by comparison of the pre-treated chicks with the control groups. Percentage inhibition of oedema was also calculated for each dose from the AUC using the equation below:
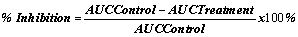
Statistical analysis
All results were plotted and analysed with GraphPad Prism 5.0 for windows (GraphPad software, San Diego, CA, USA) and analysed by two-way ANOVA followed by Bonferroni post-test analysis which recognises *p < 0.05, **p < 0.01, ***p < 0.001 as statistically significant.
Phytochemical screening
Phytochemical screening of the extracts revealed the presence of saponins, phenols, tannins flavonoids and triterpenoids in ADR and CEL. Majority of the secondary metabolites tested were found absent in ADL. Alkaloids were found to be absent in all the extracts (Table 1).
| Constituents | ADR | ADL | CEL |
|---|---|---|---|
| Saponins | + | - | + |
| Phenols | + | - | + |
| Tannins | + | - | + |
| Glycosides | - | - | + |
| Alkaloids | - | - | - |
| Flavonoids | + | - | + |
| Triterpenoids | + | + | + |
| Sterols | - | + | + |
Table 1: Phytochemical screening of extracts.
Minimum inhibitory concentration (MIC)
The extracts demonstrated broad spectrum antibacterial and antifungal activity against the test microorganisms. ADL and ADR showed MIC values within the ranges of 12.5 to 50 mg/mL whiles CEL was within ranges of 25 to 50 mg/mL (Table 2).
| Organism | Minimum Inhibitory Concentration | ||||
|---|---|---|---|---|---|
| CEL | ADL | ADR | Ciprofloxacin µg/mL | Ketoconazole mg/mL |
|
| mg/mL | |||||
| E. coli | 25.00 | 12.5 | 12.5 | 5.0 | NA |
| S. aureus | 50.0 | 50.0 | 50.0 | 10.0 | NA |
| P. aeruginosa | 50.0 | 50.0 | 25.0 | 5.0 | NA |
| S. pyogenes | 50.0 | 50.0 | 25.0 | 5.0 | NA |
| C. albicans | 50.0 | 50.0 | 25.0 | NA | 10.0 |
Table 2: Minimum inhibitory concentration (MIC) of extracts (ADR, ADL and CEL) and reference antibiotics.
Antioxidant activity
The extracts demonstrated very good antioxidant activity at the concentrations tested. CEL, demonstrated the highest antioxidant activity amongst the extracts with the lowest IC50 value. (Table 3 and Figure 1). Total antioxidant capacity and total phenolic content revealed an increase in these parameters with increase in extract concentration (Figures 2 and 3).
| Extract / Compound | IC50(µg/mL) |
|---|---|
| α- tocopherol | 4.176 |
| ADR | 627.3 |
| ADL | 669.9 |
| CEL | 146.9 |
Table 3: Antioxidant activity of α- tocopherol and extracts (ADR, ADL and CEL).
Anti-inflammatory activity
ADR, ADL and CEL demonstrated anti-inflammatory activities at the doses used. All the doses of extracts (ADL, ADR and CEL) tested at 30 and 300 mg/kg body weight exhibited significant (p < 0.001) activity over the course of duration of the experiment than the dose of 100 mg/ kg (Figure 4).
Figure 4: Influence of aspirin and extracts (ADR, ADL and CEL) on carrageenan-induced inflammation in chicks. A: Effect of A. difformis leaf extract on time-course curve; B: Effect of A. difformis leaf extract on auc of carrageenan induced oedema; C: Effect of A. difformis root extract on timecourse curve; D: Effect of A. difformis root extract on auc of carrageenan induced oedema: E: Effect of C. esculentus leaf extract on time-course curve; F: Effect of C. esculentus leaf extract on auc of carrageenan induced oedema; ADL: A. difformis Leaf Extract; ADR: A. difformis Root Extract; CEL: C. esculentus Leaf Extract; *p < 0.05, ***p < 0.001 Compared to Vehicle-Treated Group (Two-way ANOVA followed by Bonferroni’s post hoc test); N = 5, Values are means ± SEM.
Studies conducted on the roots and leaves of A. difformis and leaves of C. esculenta showed that these plants possess some pharmacological or biological properties [5,21]. The preliminary phytochemical screening of the roots and leaves of A. difformis revealed the presence of saponins, tannins, flavonoids and phenols in the roots of the plant while these secondary metabolites were absent in the leaves. The leaves were found to have triterpenoids and sterols present. Alkaloids were not observed in all the extracts. These findings confirm earlier research conducted on these plant parts [5,21]. The leaves of C. esculenta revealed the presence of phenols, tannins, glycosides, flavonoids, triterpenoids and sterols.
All the plant extracts demonstrated antimicrobial activity against both Gram-negative and Gram-positive bacteria as well as the fungus (C. albicans). ADR exerted better antimicrobial activity than ADL as reported by Abah et al. [21] and Oyetayo [5]. The antimicrobial activity can be attributed to the secondary metabolites present in the extracts [22] which were present in ADR and CEL.
The DPPH free radical scavenging, total antioxidant activity and total phenolic content methods were used in assessing the antioxidant activity of the extracts due to high speed and sensitivity and because it is imperative to use more than one method to evaluate antioxidant nature or capacity of plant materials due to the complex nature of the phytochemicals present in them [23]. The IC50 value indicates the extent of antioxidant activity with lower values indicating potent antioxidant activity. CEL had the highest radical scavenging activity as compared to the other two extracts with ADL lowest antioxidant property (Table 3). Antioxidant activity has been attributed to the presence of phenolic compounds in plants which were present in ADR and CEL [3,24,25]. The results from the total phenolic content revealed increasing phenolic content with increase in extract concentration. This was also similar with the total antioxidant capacity. Antioxidant activity can therefore be said to increase with increase in phenolic content which supports our findings.
The anti-inflammatory studies revealed that ADR, ADL and CEL possess significant anti-inflammatory activity. The time course curve shows a reduction in oedema in ADR, ADL and CEL-treated rats when compared to the control. This implies that the extracts possess significant inhibitory effects on preformed mediators such as histamine and serotonin which are involved in the initial phase of the acute inflammatory process. The inhibitory effect of the extracts extended to the later phase implicating the role of arachidonic acid metabolites as well as polymorphonuclear cells in the inflammatory process [26]. A. difformis and C. esculentus therefore exhibit prophylactic efficacy against inflammation in chicks. The anti-inflammatory activity could be due to the presence of steroids in the extracts. Steroids are known to reduce inflammation by preventing phospholipase A2 from hydrolysing arachidonic acid from phospholipids in the cell membrane. This eventually results in a reduction in prostaglandins and thromboxanes which are important for inflammatory effects Aspirin which was used as a control is a non-steriodal anti-inflammatory drug (NSAID) which inhibits cyclooxygenase enzyme (COX-2) hence inhibiting prostaglandin and thromboxane [27]. The phytochemical screening revealed the presence of steroids in the extracts which could have been responsible for the anti-inflammatory activity. ADR, ADL and CEL at the doses tested demonstrated better anti-inflammatory activity than aspirin. The mechanism of action could be due to the inhibition of the arachidonic pathway. There is need to isolate the bioactive agents responsible for the above observed biological activities. These findings could therefore justify the folkloric use of A. difformis and C. esculenta.
Methanol extracts of A. difformis and C. esculenta possess broad spectrum antimicrobial and antioxidant activity. Extracts (ADR, ADL and CEL) exhibited significant anti-inflammatory activity.
Authors acknowledge the technical support of Mr. Francis Amankwah, Department of Pharmaceutics (Microbiology Section) and Mr. Thomas Ansah in the Department of Pharmacology, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
Authors declare no competing interests.