Journal of Geology & Geophysics
Open Access
ISSN: 2381-8719
ISSN: 2381-8719
Short Communication - (2025)Volume 14, Issue 4
Large amounts of H2 can be stored and quickly extracted again using H2 geo-storage, which has been identified as a crucial technology. H2 storage in regionally extensive sedimentary geologic formations that could offer significant storage space is one approach that is currently being investigated. Structural trapping, in which a caprock prevents the buoyant H2 from ascending due to capillary forces, is the process that holds the gas in the subsurface. It is crucial to determine how much H2 can be trapped within a structure under specific geo-thermal conditions. Thus, this structural trapping capability is evaluated, and it is shown that a maximum amount of H2 may be held at a depth of 1100 m, which is the best storage depth for H2.
The fundamental obstacle to adopting an industrial-scale hydrogen economy is the inability to store huge amounts of hydrogen. Hydrogen geo-storage (UHS) has been suggested as a viable solution. In UHS, H2 can be continuously injected into subsurface geologic formations and then removed. Sedimentary reservoirs, which are common and have large storage capacities, are one target formation currently under investigation. These reservoirs are also thought to be suitable for CO2 Geo- Sequestration (CGS) and can typically hold natural gas reserves, which have been used in industrial production for a long time. Geologists refer to the seal layer as caprock, and it is impermeable, storing the buoyant gases (H2, CH4, and CO2).
Although a caprock is technically a sedimentary rock, it has a very low permeability. It is also porous. The high capillary entry pressure (Pc,e) of the caprock, which is again connected to the extremely small pores in the caprock, is the cause of the buoyant gases' inability to percolate into the caprock [1].
However, if the buoyancy pressure is greater than Pc,e, the buoyancy forces will prevail over the opposing capillary forces, causing gas to migrate through the caprock and ascend. Equation (1), a buoyancy force-capillary force balance, can be used to calculate this.
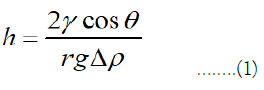
Where Δρ is the difference between the density of water (ρw) and the density of gas (ρg), h is the height of the column of gas that can be completely immobilized beneath the caprock, g is the gravitational constant, γ is the gas-water interfacial tension, and θ is the angle between the water, rock, and gas. Since Δρ, γ and θ all vary greatly with depth; it has been previously shown that in the context of CGS, h fluctuates with storage depth [2]. As a result, there is a lower depth limit below which CO2 cannot be structurally trapped and permanently stored (although it is important to note that below 15,000 m, CO2 is heavier than formation brine and sinks spontaneously due to gravitational forces deep into the reservoir). Additionally, there is a maximum depth at which CO2 can be held that is optimal for storage. Here, it is postulated that UHS also has an ideal storage depth for storing the greatest amount of hydrogen, even though hydrogen would always float because of its high volatility in a geologic reservoir.
It is obvious that three factors in equation (1), namely Δρ, γ and θ are influenced by pressure, temperature, and therefore depth. A hydrostatic gradient of 10 MPa/km and a geothermal gradient of 30 K/km are assumed for the analysis in order to approximate typical subsurface conditions [3]. Due to the fact that H2 is a very highly compressible gas, the temperature and pressure ranges that are crucial in UHS (300–360 K and 0.1–20 MPa) only have a negligible impact on H2 density (H2). With rising pressure but falling sharply with rising temperature, the H2-water interfacial tension is reduced. In addition, θ increases relatively strongly with depth (mostly due to the increasing pressure). With increasing depth, the H2 column height h falls monotonically [4].
H2 geo-storage is being investigated as a practical and affordable way to enable the ubiquitous storage of huge amounts of H2. A caprock acts as a geologic seal that prevents the passage of H2 because of its high capillary entry pressure. It is the major proposed storage method for the buoyant H2 in the subsurface. It is evident that H2 can migrate upwards, though, if buoyancy forces are greater than capillary forces, which again rely on the amount of H2 stored (exactly the vertical H2 column height h).
Citation: Chen P (2025) An Overview on Hydrogen (H2) Geological Storage Technique. J Geol Geophys. 14.1243.
Received: 02-Sep-2025, Manuscript No. JGG-22-20503; Editor assigned: 06-Sep-2025, Pre QC No. JGG-22-20503 (PQ); Reviewed: 20-Sep-2025, QC No. JGG-22-20503; Revised: 27-Sep-2025, Manuscript No. JGG-22-20503 (R); Published: 04-Oct-2025 , DOI: 10.35841/2381-8719.25.14.1238
Copyright: © 2025 Chen P. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.