Advanced Techniques in Biology & Medicine
Open Access
ISSN: 2379-1764
ISSN: 2379-1764
Research Article - (2021)Volume 9, Issue 6
Strategy of assignment influences the accuracy of CT-image based bone material properties of finite element model. An in-house assigning package was produced and compared the predicted results (stress and strain) with Bonemat 3.2 using a validated TCVO finite element model in physiological loading condition. The stress had local consistency and much variability was addressed including the strain prediction. May be different algorithms between assigning packages was the reason. Optimizing the algorithm and more tests are needed further to improve the accuracy of assigning bone material properties for ABAQUS users.
Material Assignment Strategy, Finite Element Model, ABAQUS user
Trabecular bone is characterized by anisotropy and heterogeneity which effects the mechanical performance of the whole bone [1]. In order to create more realistic finite element models, it is essential to describe the properties of trabecular bone correctly for drawing meaningful results. It is possible that the site-specific elastic modulus can be calculated from bone mineral density (BMD) derived from Computed Tomography (CT) image [2], the elastic modulus-density relationships applied in finite element analysis is a power equation that is adopted from empirical formulas [3,4].
However, we focused on the practical strategy of such elastic modulus-density relationships when utilizing the finite element software ABAQUS. This is the material mapping strategy that influences the accuracy of CT-based finite element analysis for bones [5-7]. It is available to perform the bone material assignment for an ABAQUS input file (Inp) with commercial medical processing software, such as Mimics (Materialise, Belgium), and a publicly available software named Bonemat created by researchers at the Istituto Ortopedic Rizzoli in Bologna, Italy, to help with accurate material assignment for a finite element mesh, which is updated regularly and can be downloaded freely. Moreover, the stable version of Bonemat 3.2 has a main improvement that can be currently compatible with ABAQUS models, so the uses do not have to convert their finite element meshes into a compatible format, before material properties can be assigned. The algorithm used by Bonemat 3.2 to calculate the material properties of bone is from CT series. First, convert the CT scan voxels to modulus values, and then perform the linear interpolation and numerical integration to find the modulus for each element volume [7]. However, the limitations still exist, which can be not satisfied with our research demands fully.
An in-house software we have created, and aims to address the limitations of such as Bonemat for ABAQUS users. It has been written in Matlab (MathWorks Software Foundation, USA) and applies the similar calculations as Bonemat, but the mesh data input format is an ABAQUS input file. Furthermore, the program has the added functionality that can convert to Binarized image from CT image when extracting the geometry of bone, calculate the coordinates of femoral head center. Connecting better with Mimics is more benefit from using our material assignment software package for ABAQUS users.
The purpose of the present study was to: (1) assess equivalence between the in-house program and the BoneMat3.2 version by evaluating the mechanical parameters (von Mises stress and principal strain) using our validated transtrochanteric curved varus osteotomy (TCVO) finite element model.
Material assignment software preparation
The stable version 3.2 of Bonemat was downloaded from. And the parameters used to assign the material properties were descripted in (Table 1). Output file can be input to ABAQUS as a model file directly with assigned materials. The parameters used for inhouse assigning package were summarized in (Table 2), briefly, the parameters used for converting HU to radiographic CT density (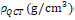 (Eq. (1)) and from to Ash density
(Eq. (1)) and from to Ash density 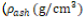 (Eq. (2)) were based on reported values for the femur . The apparent density which was calculated from Ash density with a ratio of 0.6 was converted to elastic modulus (Eq. (3)) .
(Eq. (2)) were based on reported values for the femur . The apparent density which was calculated from Ash density with a ratio of 0.6 was converted to elastic modulus (Eq. (3)) .

Where Eq. (1), Eq. (2), Eq (3) were used for calculating elastic modulus, which were different from Bonemat in which converting to elastic modulus from Ash density directly.
Simulation of finite element TCVO model
The intact femur 3D model was extracted from CT images (Figure. 1a). After that, four TCVO models with 3°,6°,9°,12° were simulated in the study (Figure. 1b). Validated finite element TCVO models was used from our previous report [11]. The mesh type was C3D4 with an element size of 1 mm for the two osteotomy parts before assigned. Then meshed parts were imported into in-house program and Bonemat for the CT-based isotropic heterogeneous property, separately. Three INP files were output consisting of nodes and elements of head, shaft and both in each of files. Just two of the three files which contained head and shaft separately were assigned, then the material property of head and shaft were input the third INP file using Notepad, respectively. The third INP file that contained nodes, elements and material property of both head and shaft was input as a model file in ABAQUS. And the interfaces of two osteotomy parts were targeted as the region of interest (ROI). The bonding interaction of the interfaces was performed for simulating the finish bone-healing stage after osteotomy. For boundary condition, the distal femur was constrained totally with a physiological condition that simulation the single-legged stance (Figure. 1c), corresponding to the physiological configuration in the selected phase of gait
Figure 1: Reduction of TCVO finite element model, a, intact femoral 3D model, b, transtrochanteric curved varus osteotomy (TCVO), c, finite element model of TCVO.
Evaluation for the ROI
Predicted values (von Mises stress, principal strain) calculated by each package were compared. In addition, in order to estimate the local error that can affect the prediction, the peak error, and average error (root mean square (RMS) error) were computed, Bonemat 3.2 was regarded as a reliable setting base data.
Speed comparison
The time taken to calculate the finite element models using the different material assigning packages was assessed, each model was measured 3 times, material assignment was performed on a Windows 7 PC with a 64- bit operating system, with four CPUs, 8 GB of RAM, and an Intel Core i5 processor.
In this study, the in-house material assignment package was used as Method 1and the Bonemat 3.2 as Method 2. The calculating time of method 1 for assigning was about 30 minutes and method 2 spent about 15 seconds. The max von Mises stress value had no significant difference in 0°, 6° models in upper interface and 0°, 3° models in lower interface, other models had a significant difference and disorganized between two methods (Figure. 2a, b), moreover, the RMSE was calculated for both absolute and percentage values that were 1.29 and 53.3. The distribution of von Mises stress was similar for both methods, especially, in 0°, 3°, 6° models (Figure. 3).
Figure 2: Predicted values of stress and strain of ROI by two methods, a, max von Mises stress in upper interface, b, max von Mises stress in lower interface, c, max principal strain in upper interface, d, max principal strain in lower interface.
Figure 3: Predicted stress distribution of ROI by two methods.
The max principal strain value was higher in 0°, 3° models and lower in 6°, 9°, 12° models for method 1 in both interfaces (Figure. 2c, d), moreover, the RMSE was calculated for both absolute and percentage values that were 467 and 77.6 (Table 5). The distribution of principle strain was similar in 0°, 3° models for both methods, however, comprehensive distribution displayed in 3°, 6°, 9° models for method 1 compared to method 2 (Figure. 4).
Figure 4: Predicted strain distribution of ROI by two methods.
Purpose of the present study was to evaluate to what extent the average strategy for assignment of material properties of bone model from CT images with an in-house material assigning package compared to the stable release Bonemat 3.2 in the level of stress and strain [8].
Predicted results showed that the stress and strain in ROI were not as well as expected. Significant difference was addressed in most models, the higher degree of TCVO, the higher variability of two methods was. Especially, in terms of the strain, not only the variability, but also disorganized between models (Figure 2). For explanation, may be the difference of density-modulus relationships applied in each assigning package, although the density-modulus calculating algorism (Tables 1 and 2) was different, the stress prediction of two methods was comparable in lower osteotomy degree (Figure 2a, b), and the stress distribution was similar in those models (Figure. 3). However, the strain distribution of method 1 was more harmonious and smoothy than method 2 with locally concentrated [9-11].
For stress, the RMSE was calculated with both absolute and percentage values that were 1.29 and 53.3, for max principal strain, the RMSE was calculated with both absolute and percentage values that were 467 and 77.6. Fulvia Taddei reported that stress prediction with RMSE=2.5 as the average absolute error, and strain prediction with RMSE=517 as the average absolute error by using Bonemat V3 comparing with the experimental test data [6]. For the local peak error, there was 64.4% of the Maximum Bonematbased stress, which was higher than the report that was 26.5% of the Maximum measured stress. There was 128% of the Maximum Bonemat-based predictive strain, which was lower than the report that exceeded 200% of the Maximum measured strain [12].
On the other hand, the workflow advantages of the in-house program and added functionality (conversion to Binarized image from CT image, calculation of coordinates of femoral head center) connecting better with Mimics are more benefited from using our material assignment package for ABAQUS users, however, our package took significantly longer (30 mins) to perform the material assignment calculations compared with the Bonemat software (10s) in the present study. The time to assign the finite element model depends on the element volumes.
On the other hand, the workflow advantages of the in-house program and added functionality (conversion to Binarized image from CT image, calculation of coordinates of femoral head center) connecting better with Mimics are more benefited from using our material assignment package for ABAQUS users, however, our package took significantly longer (30 mins) to perform the material assignment calculations compared with the Bonemat software (10s) in the present study. The time to assign the finite element model depends on the element volumes. Bonemat 3.2 strategy on all indicators, both local and global, should depend on the randomly selected specimen experiments. Further research is needed for more reliable and comparable results.
In summary, although this in-house material assignment package had an acceptable predictive result compared with BnoeMat 3.2 version in local view, however, much variability was addressed, thus, further detailed test and discussion was needed for the accuracy of strategy for material assignment from CT data in finite element analysis.
None.
No conflict of interest is declared.
No funding was received for this.
Citation: Feng W, Zhang H, Kang Z (2021) An in-house Approach for Assigning Bone Material Properties of Finite Element Models Applied in ABAQUS. Adv Tech Biol Med. 9:303. doi: 10.4172/2379-1764.1000303
Received: 30-May-2021 Accepted: 15-Jun-2021 Published: 22-Jun-2021 , DOI: 10.35248/2379-1764.21.9.303
Copyright: © 2021 Hang-hang Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.