Research Article - (2023)Volume 8, Issue 4
The SARS-CoV-2 is the causative agent of COVID-19, a disease that was declared a pandemic by the World Health Organization (WHO), at the beginning of 2020. Various authors have pointed out that morbidity and mortality as well as the economic consequences of a pandemic are influenced by factors such as the susceptibility of the population to infection, the severity of the disease, transmissibility and routes of transmission. It was of particular interest in this work to focus on the importance of the back of the hand during the transmission process of SARS- CoV- 2. The expression, as well as the functionality of the ACE2 receptors on the back of the hand was demonstrated by immune-histochemical techniques and the implications of these findings in the indirect transmission of the virus were discussed. We conclude that contact with the skin for example, through the fist salute, contributes to the increase in transmissions, in such a way that we suggest that this practice be avoided as a protection measure.
COVID-19; SARS-CoV-2; Receptors ACE2; Transmission; Immunohistochemical; Back of the hand
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; ACE2: Angiotensin-Converting Enzyme 2; RBD: Receptor-Binding Domain; FP: Fusion Peptide; MERS-CoV: Middle East Respiratory Syndrome; ARDS: Acute Respiratory Distress Syndrome; CSS: Cytokine Storm Syndrome; NTD: N-terminal Domain; RBM: Receptor-Binding Motif; HR1 and 2: Hepta-Repeat Domains 1 and 2; TM: Transmembrane; CP: Cytoplasmic; REC: Research Ethics Committee
The coronavirus that causes severe acute respiratory syndrome, type 2 Severe Acute Respiratory Syndrome Coronavirus 2 (SARS- CoV-2), is the causative agent of COVID-19. This virus belongs to the Coronaviridae family which includes the SARS-CoV and the virus that causes Middle East Respiratory Syndrome (MERS-CoV), all lof them, causing severe clinical syndromes [1,2]. SARS-CoV-2 has a high transmission rate, mainly by aerosols and close contact and the respiratory tract has been recognized as the main route of entry into the body, through infected people or through contact with contaminated surfaces, where the pathogen remains active for hours. Furthermore, it is well recognized that infected people have mild to moderate respiratory symptoms, after an incubation period of up to 14 days, others face severe symptoms that ultimately lead to Acute Respiratory Distress Syndrome (ARDS), associated with a Cytokine Storm Syndrome (CSS) [3,4].
The SARS-CoV-2 receptor Angiotensin-Converting Enzyme 2 (ACE2), which is found in the human respiratory system and with higher expression in older adults than in younger adults is known to is the anchor point and pathogenicity of this virus [5]. In vitro studies support the interaction between ACE2 and the protein “Spike (S)” or SARS-CoV-2 spike. The surface glycoprotein S of coronaviruses, which mediates attachment to and entry into target cells, is composed of two subunits, S1 and S2. The S1 subunit contains an N-terminal Domain (NTD) and a Receptor-Binding Domain (RBD) encompassing the Receptor-Binding Motif (RBM). The S2 subunit contains a Fusion Peptide (FP), Hepta-Repeat Domains 1 (HR1) and Hepta-Repeat Domains 2 (HR2) and a Transmembrane (TM) and Cytoplasmic (CP) domain [6-8].
After S1 binds to a membrane receptor, FP inserts into the cell membrane to promote fusion with the viral membrane, a process that depends on proteolytic cleavages at the S1/S2 site to separate S1 and S2 and into the S2 site to generate a mature FP [9,10].
Although the ACE2 receptor is typical of type II pneumocytes and bronchial hair cells, it is not exclusive to the respiratory system since there are other tissues and organs that express said receptor, such as the heart, kidneys, liver, intestines, oral cavity, nasal, brain, thyroid, stomach, reproductive systems, lungs, pancreas, eyes and skin (Figure 1) [11].
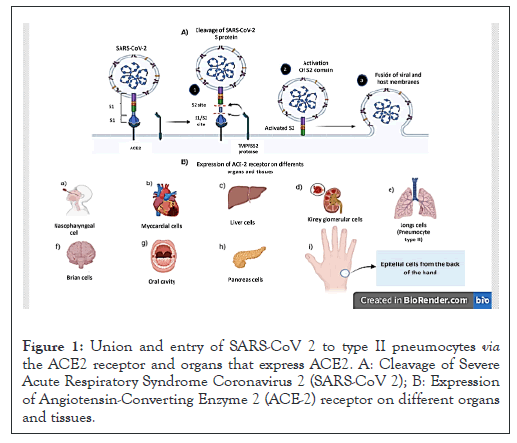
Figure 1: Union and entry of SARS-CoV 2 to type II pneumocytes via the ACE2 receptor and organs that express ACE2. A: Cleavage of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV 2); B: Expression of Angiotensin-Converting Enzyme 2 (ACE-2) receptor on different organs and tissues.
Regarding the expression of ACE2 in the skin, it has been shown that it is present in the basal cell layer of the epidermis, extending to the basal cell layer of hair follicles. Smooth muscle cells surrounding sebaceous glands are also positive for this receptor. In addition, a strong granular staining pattern for ACE2 has been seen in organs that express ACE2 in cells of the eccrine glands, however, little is known about the distribution of SARS-CoV-2 in different regions of the skin [12].
In the literature it has been reported that the skin is not uniform, it presents differences in its permeability, activity of the immune system and even its microbial composition. The distribution of sebaceous cells seems to be an important point, since areas rich in sebaceous glands are characterized by weaker permeability barrier characteristics mainly due to lower expression of tight junction proteins. It is for this reason that it is important to analyze specific epithelial regions, which may be more susceptible to SARS-CoV-2 infection or failing that be a site of probable transmission such is the case of the back of the hand. In our work group we analyzed the expression of ACE2 in a group of tissue biopsies from the back of the hand in order to discuss the importance of said tissue during the transmission process of SARS-CoV-2 [13].
Human tissue samples
Samples from 5 patients with a presumptive diagnosis of actinic keratosis who attended the Hospital Regional de Alta Especialidad (HRAEI), undergoing skin excision biopsy procedures for diagnostic corroboration purposes. From the samples obtained, areas of healthy skin were selected, which were used to perform immunohistochemistry. All procedures and use of tissue (anonymized) were performed in accordance with recent national ethical guidelines. The mean age of the patients was 40 to 55 years and the male: female ratio was 3:2. All the individuals included in the study signed the informed consent, with the endorsement of the Research Ethics Committee (REC) of the HRAEI.
Immuno-histochemical assay
ACE2 receptors on the back of the hand: Specimens were fixed in formalin and embedded in paraffin. Sections of 3-5 μm were processed for histopathological analysis. Immunohistochemistry was performed using the MACH 1 universal HRP-polymer detection kit (Biocare Medical, Concord, CA, USA). The skin sections were mounted on lamellae, dewaxed in xylene, hydrated with a series of alcohols graduated in water and subjected to antigen recovery. Next, endogenous peroxidases were blocked with 3% hydrogen peroxide. The sections were incubated overnight at room temperature with optimal dilution of mouse anti-ACE2 polyclonal primary antibody (R and D Systems Mexico). After incubation with the antibody, MACH1 mouse was added (Biocare Medical) and incubated for another 15 minutes. Subsequently, incubation was performed with the MACH 1 universal HRP-polyme (Biocare Medical), for 30 minutes. Next, the stain with DAB (3,3’-Diaminobenzidine Biocare Medical) solution was performed. The sections were then countered with hematoxylin. Positive immune-reactivity was manifested by brown staining. The specialized pathologist carried out the clinical interpretation of any staining, positive or negative, morphology and other histopathological criteria for the identification of ACE2 receptors. External positive controls were nasopharyngeal mucosal samples, with well-characterized levels of positive activity for ACE2, showing positive staining peaks [14,15].
Spike protein binding to ACE2 receptors on the back of the hand
On the other hand, to identify a positive binding of the spike protein to ACE2 receptors to verify pathogenicity and/or functionality of the receptors found previously, the SARS-CoV-2 (2019-nCoV) spike RBD-his recombinant protein kit (Sino Biological US Inc.) was used. The spike protein (Sino Biological) marker was used to verify the binding of the RBD site to the ACE2 receptor subsequently, incubation was performed with the polymer anti-His Tag Antibody (BioLegend USA), for 30 minutes. The sections were then countered with hematoxylin. Positive immune reactivity was manifested by brown staining. Nasopharyngeal mucosal samples, obtained from the same patients, were used as test controls.
ACE2 expression on the back of the hand
ACE2 receptors were shown to be present on epithelial and endothelial cells in samples taken from the back of the hand of the patients included in the study. Cells and membranes were stained, denoting the presence of ACE2, positive staining for ACE2 was observed in myofibroblasts and in the endothelial cell membrane, being distributed throughout the observed skin area. Cytoplasm of epithelial cells on the back of the hand also showed weak positive staining for ACE2. In skin, ACE2 was present in the basal cell layer of the epidermis extending into the basal cell layer of hair follicles as Figures 2A-2C. Smooth muscle cells surrounding the sebaceous glands were also positive for ACE2. Weak cytoplasmic staining was lost in sebaceous gland cells, as well as a strong granular staining pattern for ACE2 in eccrine gland cells as shown in Figure 2B.
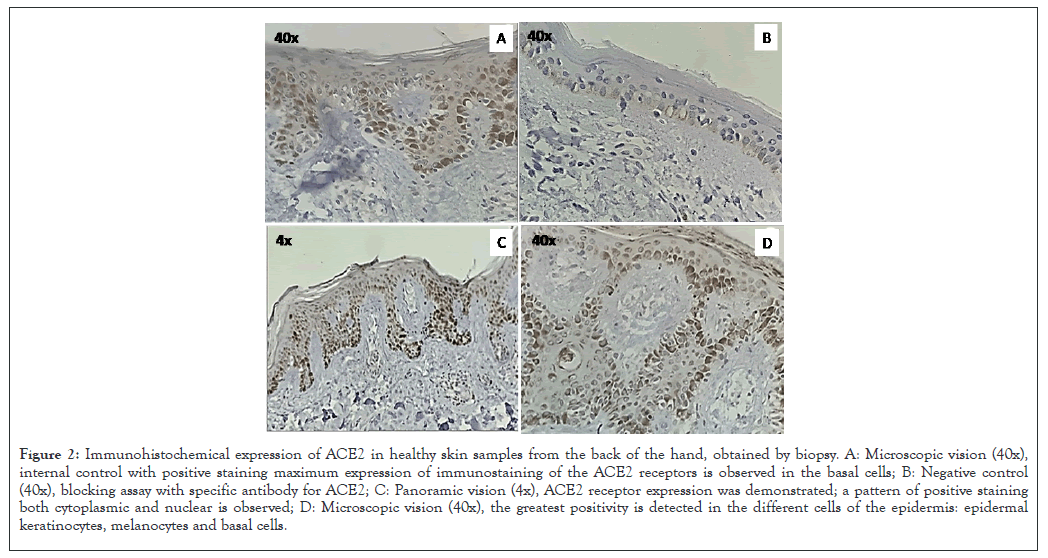
Figure 2: Immunohistochemical expression of ACE2 in healthy skin samples from the back of the hand, obtained by biopsy. A: Microscopic vision (40x), internal control with positive staining maximum expression of immunostaining of the ACE2 receptors is observed in the basal cells; B: Negative control (40x), blocking assay with specific antibody for ACE2; C: Panoramic vision (4x), ACE2 receptor expression was demonstrated; a pattern of positive staining both cytoplasmic and nuclear is observed; D: Microscopic vision (40x), the greatest positivity is detected in the different cells of the epidermis: epidermal keratinocytes, melanocytes and basal cells.
Marked ACE2 immunostaining was found in epidermis, epidermal keratinocytes, melanocytes and basal cells as shown in Figures 2A and 2B.
This indicates that, in the epithelial tissue of the back of the hand, the ACE2 receptor is expressed, turning this area into a site where SARS-CoV-2 could adhere (Figure 2).
Spike protein binding to ACE2, on the back of the hand
To reinforce the idea that the back of the hand may be a potential adhesion site for SARS-CoV-2, we decided to test the ability of the SARS-CoV-2 spike protein to bind to the ACE2 receptors on the back of the hand in hand.
For this we immune-stained the biopsies, this time using the recombinant protein spike-RBD from SARS-CoV2, which contained a histidine tag. Subsequently, we incubated with anti-his tag antibody. Positive immune-reactivity was manifested by brown staining.
The results were positive for the spike-RBD marker binding to the ACE2 receptors present on the back of the hand, previously identified in this study. In the photographs obtained as shown in Figure 3 the stratified layer of epidermis is clearly observed, in its subset with the lucid layer and part of the granular layer, as well as the spinous layer, observing granular keratinocytes, Langerhans cells and spiny keratinocytes with positive brown staining, some cells of the basal stratum are also stained, as well as some melanocytes. Intensive staining can be identified on images (Figure 3).

Figure 3: Functionality of the ACE2 receptor, after binding to the spike protein in samples from the back of the hand. A: Microscopic vision (10x), positive observation of cells of the dorsum before the union spike vs. ACE2; B: Microscopic vision (40x), positive binding spike vs. ACE2; the highest positivity is detected in different cells of the epidermis, mainly melanocytes; C: Microscopic vision (40x), internal control with positive staining; endothelial cells lining capillaries, deep superficial dermis; D: Negative control (40x), blocking assay with specific antibody for ACE2.
SARS-CoV-2, like other respiratory viruses, such as influenza and rhinoviruses, can be transmitted independently and simultaneously by several routes, either directly or indirectly. Direct transmission occurs through exhaled droplets or aerosols that reach the respiratory tract or through physical contact between a susceptible person and an infected person; indirect transmission occurs through contact with contaminated surfaces or objects and subsequent autoinoculation into mucous membranes or by serial transfer via fomites [16-18].
Although the main route of transmission is through the air, it has been suggested that indirect contact, through surfaces, including the surface of the skin, contributes considerably to the transmission of the virus. Such is the potential impact of indirect transmission that, among the most affordable non-pharmacological intervention practices to prevent and control the transmission of SARS-CoV-2 is the disinfection of inanimate surfaces and hands [19].
In this regard, since SARS-CoV-2 is remarkably stable on human skin, hand disinfection reduces the risk of indirect transmission [20]. This is particularly important in a country like Mexico, where the fist salute was established after the arrival of the omicron variant, despite the fact that it has been suggested that the skin is a potential host for SARS-CoV-2 [21].
In fact, the virus has been shown to be highly stable on the skin, suggesting its potential role in dermal transmission and the high rate of spread of the virus. The suggested transmission mechanism involves the sneezing or coughing of an infected individual, which can contaminate a surface or directly the skin of another individual. The virion can remain intact and infectious on skin (sebaceous, oily or clean skin with exposed stratum corneum), so the individual can become infected by touching their eyes, mouth or nose.
Although there is no concrete evidence of the tropism of SARSCoV- 2 in the skin, it is known that this is determined by the tissue distribution of ACE2 [22]. The studies carried out in our laboratory confirmed the expression of ACE2 on the back of the hand and its functionality after binding to the Spike protein opening the possibility of the presence of SARS-CoV-2 at this site and its potential transmissibility.
Transmissibility depends on the characteristics of the virus, including its infectivity and the environmental stress exerted on it during transmission, as well as factors attributable to the population, including the contagiousness of the infected individual, the susceptibility of the exposed individual and contact patterns between infected and exposed individuals.
Regarding viral characteristics, some studies have shown the stability of SARS-CoV-2 on skin. A study, carried out with a skin model, which replicates in vivo hand skin conditions, revealed that SARS-CoV-2 has a survival of 9.04 hours Interval of Confidence (IC 95% 7.96-10.2) and a half-life of 3.53 hours (IC 95% 3.02- 4.16) suggesting its potential role in skin transmission and in the high rate of spread of the virus. Another study reported that the virus persisted for 96 to 168 hours on the skin of pigs, with a half-life of 3.5 hours at room temperature and even longer at low temperatures. (46.8 hours a 4°C) [23].
Another study reported that the virus persisted for 96 to 168 hours on the skin of pigs, with a half-life of 3.5 hours at room temperature and even longer at low temperatures. Considering that SARS-CoV-2 is present in the skin, it has been hypothesized that, if this organ is involved in the transmission of the virus, the function of the skin barrier could be the mediator [24].
In relation to contact patterns, although it is worth noting the contribution of casual contact with contaminated surfaces, there are several limitations to its study. On one side, the frequency and manner of contact with these surfaces is variable and depends, to a large extent, on age, personal habits, type of activities, personal mobility and the level of cleanliness of the environment. On the other hand, it is difficult to distinguish between different routes of transmission, such as person-to-person transmission or autoinoculation [25].
However, as boone and gerba points out, various investigations support indirect transmission, under the following statements: (I) most respiratory viruses can survive on fomites and hands for variable periods of time; (II) fomites and hands can becoming contaminated with viruses from both natural and laboratory sources; (III) viral transfer from fomites to hands possible; (IV) the hands come into contact with the portals of entry of the viral infection; and (V) disinfection of fomites and hands interrupts viral transmission.
In Mexico, after the third wave of COVID-19, the fist salute began to be used, which involves contact of the back of the hand, between two individuals. With this, it was intended to avoid the spread of SARS-CoV-2, however, our research suggests that this greeting may be a source of transmission of this pathogen. Coupled with poor handwashing practices and the inappropriate use of disinfectants, such as alcohol-gel, indirect transmission through skin contacts with surfaces and subsequent autoinoculation, for example, by rubbing the eyes or putting fingers to the nostrils, favor the contagion roles. We conclude that contact with the skin for example, through the fist salute, contributes to the increase in transmissions, in such a way that we suggest that this practice be avoided as a protection measure.
Conceptualization: GAA, RRM, ASC, RMQ, EMR, OVL*. Methodology: MCM, GAA, OVL, EMR. Original draft preparation. RRM, ASC, RMQ, OVL Writing, review and editing. EMR, RMQ, MCM, OVL & GAA.
The Hospital Regional de Alta Especialidad Ixtapaluca and the researches who intervened in the study express their gratitude to the patient for granting permission to disclose their results. The authors thank the Hospital Regional de Alta Especialidad Ixtapaluca for the support provided in the infrastructure for the approach and development of the study. The author Martínez- Quezada R. thanks COMECYT for the support granted through the scholarship chair number CAT2022-0123.
The authors declare that they have no conflict of interest.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Valencia-Ledezma OE, Altamirano GA, Martinez MAC, Martínez-Quezada R, Rodriguez CEM, Reyes-Montes MR, et al. (2023) ACE2 Receptor Expression on the Back of the Hand: Implications in the Indirect Transmission of Sars-Cov-2. Immunogenet Open Access. 8:216
Received: 28-Nov-2023, Manuscript No. IGOA-23-28536; Editor assigned: 30-Nov-2023, Pre QC No. IGOA-23-28536 (PQ); Reviewed: 14-Dec-2023, QC No. IGOA-23-28536; Revised: 21-Dec-2023, Manuscript No. IGOA-23-28536 (R); Published: 28-Dec-2023 , DOI: 10.35248/IGOA.23.8.216
Copyright: © 2023 Valencia-Ledezma OE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.