Journal of Infectious Diseases & Preventive Medicine
Open Access
ISSN: 2329-8731
ISSN: 2329-8731
Research - (2023)Volume 11, Issue 4
Objective: Diabetes meLlitus (DM) confers a higher risk for active Tuberculosis (TB). However, there is a paucity of information about the prevalence and risk factors of Latent Tuberculosis Infection (LTBI) in DM patients. Therefore, we aimed to analyze the prevalence and infection risk factors of LTBI in DM patients in Nanshan District, Shenzhen, China.
Methods: We conducted a cross-sectional study to screen for TB in a random sample of DM patients included in basic public health management from 2019 to 2020 in two regional community health centers in the Nanshan District of Shenzhen, China. Questionnaires, Interferon-Gamma Release Assay (IGRA), and glycosylated Hemoglobin test (HbA1c) were performed on DM patients who met the criteria for inclusion. Univariate analysis and multiple logistic regression analysis were used to analyze risk factors for LTBI in DM patients.
Results: The prevalence of LTBI among DM patients was 40.47% (189/467). By univariate analysis, factors significantly associated with LTBI in DM patients were age, educational level, a previous history of tuberculosis, and recent suspected tuberculosis symptoms (P<0.05). Multiple logistic regression analysis showed that infection risk factors for LTBI in DM patients were a low educational level (OR=1.689, 95% CI:1.111-2.568; P=0.014) and a previous history of TB (OR=4.264,95% CI:1.258-14.447; P=0.020), while having recent suspected TB symptoms (OR=0.316, 95% CI:0.118-0.850; P=0.023) was protective.
Conclusion: There is a high prevalence of LTBI in DM patients in the Nanshan District of Shenzhen. A low educational level was the most prominent infection risk factor.
Diabetes mellitus; Latent tuberculosis; Prevalence; Risk factors; Interferon-gamma release tests; Glycated hemoglobin A
Latent Tuberculosis Infection (LTBI) is a state of persistent immune response to Mycobacterium Tuberculosis (MTB) antigens, but is non-infectious and does not exhibit any clinical signs of active Tuberculosis (TB) disease. 5% to 15% of the LTBI population will progress to active TB [1]. In 2020, it was estimated that approximately one-quarter of the global population was infected with MTB, of which 95% was LTBI, constituting a vast reservoir of TB infections from which active TB patients will continue to emerge [2]. It is estimated that just by treating LTBI, the incidence of active TB in South-East Asia could be reduced by 64% [3].To achieve the World Health Organization's “End TB Strategy”, the detection and management of LTBI are extraordinarily essential [4,5]. By identifying the LTBI population, TB control can be moved forward to the infected population, thereby reducing the incidence of tuberculosis disease.
It has been estimated that there were 537 million diabetic patients worldwide in 2021, and by 2030 the number of patients is expected to increase to 643 million [6]. Although conclusive data are showing that DM increases the risk of developing active TB by 3.11-fold, little is known about whether DM also increases the risk of LTBI infection in non-infected individuals [7]. A few studies have found that DM increases the risk of LTBI infection. For example, LTBI infection was observed to be more than twice as common in DM patients as in those without DM in population-based studies conducted in the United States (11.6% vs. 4.6%) and Taiwan (21.1% vs. 9.7%) [8,9]. It has been hypothesized that poor glycemic control can impair the function of the immune system, leading DM patients to be more prone to LTBI and develop into active TB [10]. Therefore, treating LTBI may have a greater impact on preventing the development of TB in DM patients than in the general population [11].
However, previous epidemiological studies on DM and LTBI are limited and the results are inconsistent, no definitive conclusion can be drawn about the association between LTBI and DM [8,9,12-18]. Moreover, there is a paucity of information about the prevalence and risk factors of Latent Tuberculosis Infection (LTBI) in DM patients. Therefore, we conducted a cross-sectional study to actively screen for TB in DM patients included in basic public health management in the Nanshan District, Shenzhen, a large new city in southern China, with the aim of accurately assessing the burden of LTBI in a population with diabetes in Nanshan District, Shenzhen, elucidating its epidemiological characteristics, exploring risk factors from non-infected status to LTBI in DM patients, and providing basic data for exploring the development of screening and intervention strategies for LTBI in a population with diabetes in Nanshan District, Shenzhen.
Data collection and study design
In this study, two regional communities in Nanshan District of Shenzhen were randomly selected: Shahe community health center and Shenzhen Bay community health center. Adult (>18 years) DM patients included in the basic public health management who had lived, worked or studied in Nanshan District for 6 months or more were recruited to screen for TB. The screening periods for Shahe Community Health Service Center and Shenzhen Bay Community Health Service Center were November to December 2019 and May to June 2022, respectively. Questionnaires, Interferon-Gamma Release Assay (IGRA), and glycosylated Hemoglobin test (HbA1c) were performed in the DM population recruited in the study. DM patients with positive IGRA results were then given chest X-ray tests. Patients with active TB and other immune system diseases were excluded. Next, qualitative sputum TB-DNA assay (Real-time polymerase chain reaction qualitative assay) was performed on patients with suspected TB symptoms or a history of TB to exclude patients with a positive result of this test. The diagnostic criteria for TB were based on WS288-2017 "Diagnostic criteria for Pulmonary Tuberculosis (PTB)" [19].
Sample size calculation
The sample size was calculated based on the standard formula for estimating individual totals 78 from cross-sectional surveys of whole-group sampling studies [20].
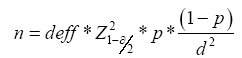
We used the global LTBI prevalence of 23%, s=23%, with deff =2, α=0.05,
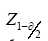 =1.96, r=0.26, d=r×p=0.0598, n=381 to estimate the
theoretical minimum sample size [21,22]. However, considering the
large sampling error of the whole group and the possible difference
between global LTBI prevalence and prevalence in the actual target
population, the sample size was floated up by 20%, yielding a
sample calculation of 457 people.
=1.96, r=0.26, d=r×p=0.0598, n=381 to estimate the
theoretical minimum sample size [21,22]. However, considering the
large sampling error of the whole group and the possible difference
between global LTBI prevalence and prevalence in the actual target
population, the sample size was floated up by 20%, yielding a
sample calculation of 457 people.
Definition of evaluation indicators
LTBI was identified in this study as those who met the following criteria: a. Positive IGRA test result; b. Exclusion of TB on chest imaging; c. Inactive TB lesions on chest imaging or self-reported history of TB with a negative qualitative TB-DNA test on sputum; d. Suspected TB symptoms with a negative qualitative TB-DNA test on sputum. Well-controlled blood glucose was defined as HbA1c ≤ 7% and poor blood glucose control was defined as HbA1c>7%. Smoking was defined as an average of more than 5 cigarettes per week over the previous year. Regular alcohol consumption was defined as the consumption of drinking alcohol for more than 6 months, at least once a week; at least 25 gram (g) of high ( ≥ 42°) white wine, or 50 g of low (<42°) white wine, or 1 bottle of beer, or 75 g of yellow wine, or 150 g of wine per drink; History of contact with TB patients was defined as working in the same office or living in the same room with TB patients during the previous 2 years [23]. Suspected TB symptoms were defined as chest pain, hemoptysis, fever, cough, and cough sputum for more than 2 weeks. The participants were categorized into five dietary structures: affluent pattern (mainly animal-based food), subsistence pattern (mainly plant-based food), nutritional pattern (both animal and plant- based food), mediterranean pattern (low intake of saturated fat and high intake of unsaturated fat, with predominantly vegetable oils). A history of dust and smoke exposure was defined as a history of exposure to asbestos, wood chips, dust, soot, sawdust, lime, or smoke in the work or living environment.
Statistical analysis
The data were statistically analyzed with SPSS 25.0 software. Continuous variables were described as mean ± standard deviation for normal distribution and median (Interquartile Range [IQR]) for non-normal distribution. Categorical variables were described by relative numbers and the student's t-test or Mann-Whitney U test was used for comparison between groups for continuous variables and the chi-square test or Fisher's exact test was used for comparison between categorical variables. Variables with P-values ≤ 0.2 in univariate analysis were included in the multiple logistic regression models [24]. The multiple analysis was performed using the conditional likelihood ratio forward stepwise method in logistic regression with the criteria of αin=0.05 and αout=0.10.
A total of 542 DM patients who met the inclusion criteria were selected for the study and sent the questionnaire. The questionnaire was returned by 499 (92%), of whom 467 underwent IGRA testing and 264 underwent HbA1c testing. The 467 individuals who underwent IGRA testing were included in the final analysis. The IGRA test results showed that the LTBI prevalence in the DM population of these two health centers in Nanshan, Shenzhen was 40.47% (189/467).
LTBI situation in DM patients and univariate analysis of factors influencing LTBI in DM patients
Effect of general demographic characteristics on LTBI in DM patients: Among the 467 study subjects, the median Body Mass Index (BMI) was 24.16 (IQR, 22.17-26.57) kg/m2; the median monthly income was 4000 (IQR, 2800-6000) yuan ($600 (IQR, $420-$900)); total annual personal medical expenses were predominantly 1001~5000 yuan (47.97%, 224/467). 52.25% (244/467) patients were male and 46.89% (234/467) patients were retired or unemployed. Most of the patients were married (90.15%, 421/467). None of these characteristics were significantly associated with LTBI (P>0.05) shown in Table 1.
Among the 467 DM patients, the median age was 65 (IQR, 55- 70) years old. Additionally, the age group of 61-80 years was predominant among them (57.17%, 267/467), and the prevalence of LTBI was highest in this age group (46.07%, 123/267). In addition, the prevalence of LTBI tended to increase with age group, with a statistically significant difference (χ2=2.425, P=0.020), but the trend chi-square test was not statistically significant (χ2=3.727, Ptrend=0.054) shown in Table 1.
| Variable | IGRA+ (n=189,40.47%) N(%) | IGRA- (n=278,59.53%) N(%) | Total (n=467,100%) N(%) | χ2 | P value |
|---|---|---|---|---|---|
| Gender | 2.425 | 0.119 | |||
| Male | 107 (43.85) | 137 (56.15) | 244 (52.25) | ||
| Female | 82 (36.77) | 141 (63.23) | 223 (47.75) | ||
| Age, years | 9.865 | 0.020a | |||
| 3.727 | 0.054b | ||||
| 18~40 | 3 (18.75) | 13 (81.25) | 16 (3.43) | ||
| 41~60 | 56 (35.00) | 104 (65.00) | 160 (34.26) | ||
| 61~80 | 123 (46.07 | 144 (53.93) | 267 (57.17) | ||
| ≥81 | 7 (29.17) | 17 (70.83) | 24 (5.14) | ||
| BMI (kg/m2) | 3.219 | 0.359 | |||
| <18.5 | 6 (33.33) | 12 (66.67) | 18 (3.85) | ||
| 18.5~23.9 | 91 (45.05) | 111 (54.95) | 202 (43.25) | ||
| 24~27.9 | 64 (36.99) | 109 (63.01) | 173 (37.04) | ||
| ≥28 | 28 (37.84) | 46 (62.16) | 74 (15.85) | ||
| Marital status | 0.015 | 0.903 | |||
| Married | 170 (40.38) | 251 (59.62) | 421 (90.15) | ||
| Unmarried/divorced/Widowed | 19 (41.30) | 27 (58.70) | 46 (9.85) | ||
| Occupation | 1.965 | 0.854 | |||
| Agriculture, forestry, animal husbandry and fishery production workers | 11 (40.74) | 16 (59.26) | 27 (5.78) | ||
| Enterprise and factory workers | 22 (44.00) | 28 (56.00) | 50 (10.71) | ||
| Merchandise and service workers | 18 (38.30) | 29 (61.70) | 47 (10.06) | ||
| Staff | 13 (32.50) | 27 (67.50) | 40 (8.57) | ||
| Retired, unemployed | 93 (42.47) | 126 (57.53) | 219 (46.9) | ||
| Others | 32 (38.10) | 52 (61.90) | 84 (17.99) | ||
| Educational level | 3.937 | 0.047a | |||
| Junior high school or below | 93 (45.59) | 111 (54.41) | 204 (43.68) | ||
| Junior high school above | 96 (36.50) | 167 (63.50) | 263 (56.32) | ||
| Average monthly income (yuan) | 0.635 | 0.728 | |||
| ≤ 2500 | 47 (41.96) | 65 (58.04) | 112 (23.98) | ||
| 2501~4999 | 60 (42.25) | 82 (57.75) | 142 (30.41) | ||
| ≥ 5000 | 82 (38.50) | 131 (61.50) | 213 (45.61) | ||
| Total annual expenditure on personal medical expenses (yuan) | 0.275 | 0.871 | |||
| ≤1000 | 31 (39.24) | 48 (60.76) | 79 (16.92) | ||
| 1001~5000 | 89 (39.73) | 135 (60.27) | 224 (47.97) | ||
| >5000 | 69 (42.07) | 95 (57.93) | 164 (35.12) |
Note: aStatistically significant; χ2 (categorical) two-sided; P value<0.05; bTrend χ2 test was used for analysis; DM: Diabetes Mellitus; LTBI: Latent Tuberculosis Infection.
Table 1: LTBI status of DM patients stratified by general demographic characteristics.
While the majority of the study population was educated beyond junior high school level (56.32%, 263/467), LTBI prevalence was higher in those whose educational level was junior high school or below (45.59%, 93/204; χ2=3.937, P=0.047) (Table 1).
Effect of behavioral lifestyle characteristics on LTBI in DM patients: Among the study subjects, 72.38% (338/467) never smoked, 91.86% (429/467) had no history of regular alcohol consumption in the past year, 70.66% (330/467) exercised daily, 79.87% (373/467) consumed meat, eggs and dairy foods more than 3times/week, 88.65% (414/467) consumed vegetables and fruits more than 3 times/week, 93.79% (438/467) had no history of exposure to dust or fumes. The sleep profile of DM patients was dominated by 6-8 hours of sleep (58.46%, 273/467) and the dietary structure of DM patients was dominated by nutritional patterns (both animal and plant-based food) (60.39%, 282/467). LTBI prevalence was not statistically associated with any of these characteristics (P>0.05) shown in Table 2.
| Variable | IGRA+ (n=189,40.47%) N(%) | IGRA- (n=278,59.53%) N(%) | Total (n=467,100%) N(%) | χ2 | P value |
|---|---|---|---|---|---|
| Smoking status | 1.778 | 0.62 | |||
| Never smoked | 131 (38.76) | 207 (61.24) | 338 (72.38) | ||
| Used to smoke, now do not | 7 (41.18) | 10 (58.82) | 17 (3.64) | ||
| Yes, but not every day | 29 (43.94) | 37 (56.06) | 66 (14.13) | ||
| Yes, daily | 22 (47.83) | 24 (52.17) | 46 (9.85) | ||
| Did you drink alcohol regularly in the past year? | 2.539 | 0.111 | |||
| No | 169 (39.39) | 260 (60.61) | 429 (91.86) | ||
| Yes | 20 (52.63) | 18 (47.37) | 38 (8.14) | ||
| Sleep time (hours/day) | 6.145 | 0.105 | |||
| ≥8 | 38 (48.10) | 41 (51.90) | 79 (16.92) | 3.219 | 0.359 |
| 6~8 | 113 (41.39) | 160 (58.61) | 273 (58.46) | ||
| 4~6 | 37 (34.58) | 70 (65.42) | 107 (22.91) | ||
| Lack of sleep/ poor sleep quality | 1 (12.50) | 7 (87.50) | 8 (1.71) | ||
| Frequency of going to crowded places | 0.236 | 0.889 | |||
| <2 times/week | 51 (40.48) | 75 (59.52) | 126 (26.98) | ||
| 2~3 times/week | 67 (39.18) | 104 (60.82) | 171 (36.62) | ||
| >3 times/week | 71 (41.76) | 99 (58.24) | 170 (36.40) | ||
| Exercise situation | 0.09 | 0.765 | |||
| Not exercise daily | 54 (39.42) | 83 (60.58) | 137 (29.34) | ||
| Exercise daily | 135 (40.91) | 195 (59.09) | 330 (70.66) | ||
| Type of dietary structure | 2.791 | 0.425 | |||
| Mediterraneann pattern | 2 (66.67) | 1 (33.33) | 3 (0.64) | ||
| Affluent pattern | 11 (29.73) | 26 (70.27) | 37 (7.92) | ||
| Subsistence model | 61 (42.07) | 84 (57.93) | 145 (31.05) | ||
| Nutritional pattern | 115 (40.78) | 167 (59.22) | 282 (60.39) | ||
| Frequency of meat, egg and dairy food intake | 2.019 | 0.155 | |||
| <3 times/week | 32 (34.04) | 62 (65.96) | 94 (20.13) | ||
| >3 times/week | 157 (42.09) | 216 (57.91) | 373 (79.87) | ||
| Frequency of intake of vegetables and fruits | 0.53 | 0.467 | |||
| <3 times/week | 19 (35.85) | 34 (64.15) | 53 (11.35) | ||
| >3 times/week | 170 (41.06) | 244 (58.94) | 414 (88.65) | ||
| Dust fume exposure frequency | 3.113 | 0.374 | |||
| None | 180 (41.10) | 258 (58.90) | 438 (93.79) | ||
| 1 times/week | 1 (12.50) | 7 (87.50) | 8 (1.71) | ||
| 2~3 times/week | 2 (28.57) | 5 (71.43) | 7 (1.50) | ||
| >3 times/week | 6 (42.86) | 8 (57.14) | 14 (3.00) |
Note: aStatistically significant; χ2(categorical) two-sided; P value <0.05; DM: Diabetes Mellitus; LTBI: Latent Tuberculosis Infection.
Table 2: LTBI status of DM patients stratified by behavioral lifestyle characteristics.
Effect of home environment characteristics on LTBI in DM patients: Among the study subjects, 6.21% (29/467) lived alone, and 6.64% (31/467) had damp indoor housing. The living area of DM patients was predominantly 50-99 m2 153 (44.97%, 210/467). Most DM patients had housing ventilation for more than 4 hours/ day (58.03%, 271/467) and used it in the home for less than 6 hours/day (66.60%, 311/467). None of these characteristics were significantly associated with LTBI (P>0.05) (Table 3).
| Variable | IGRA+ (n=189,40.47%) N(%) | IGRA- (n=278,59.53%) N(%) | Total (n=467,100%) N(%) | χ2 | P value |
|---|---|---|---|---|---|
| Living alone | 0.083 | 0.774 | |||
| Yes | 11 (37.93) | 18 (62.07) | 29 (6.21) | ||
| No | 178 (40.64) | 260 (59.36) | 438 (93.79) | ||
| Living area(m2) | 1.096 | 0.578 | |||
| <50 | 34 (35.79) | 61 (64.21) | 95 (20.34) | ||
| 50~99 | 88 (41.90) | 122 (58.10) | 210 (44.97) | ||
| ≥ 100 | 67 (41.36) | 95 (58.64) | 162 (34.69) | ||
| Housing natural light exposure time (hours/day) | 1.012 | 0.603 | |||
| ≤3 | 90 (42.45) | 122 (57.55) | 212 (45.4) | ||
| 4~6 | 77 (39.90) | 116 (60.10) | 193 (41.33) | ||
| >6 | 22 (35.48) | 40 (64.52) | 62 (13.28) | ||
| Housing ventilation time (hours/day) | 3.246 | 0.355 | |||
| <1 | 7 (30.43) | 16 (69.57) | 23 (4.93) | ||
| 1~2 | 38 (48.10) | 41 (51.90) | 79 (16.92) | ||
| 2~3 | 39 (41.49) | 55 (58.51) | 94 (20.13) | ||
| ≥ 4 | 105 (38.75) | 166 (61.25) | 166 (61.25) | ||
| Exhaust fan use in housing (hours/day) | 4.216 | 0.122 | |||
| <6 | 136 (43.73) | 175 (56.27) | 311 (66.60) | ||
| 6~12 | 42 (33.33) | 84 (66.67) | 126 (26.98) | ||
| ≥ 12 | 11 (36.67) | 19 (63.33) | 30 (6.42) | ||
| Humidity in the housing room | 0.864 | 0.353 | |||
| Humid | 15 (48.39) | 16 (51.61) | 31 (6.64) | ||
| Dry | 174 (39.91) | 262 (60.09) | 436 (93.36) |
Note: aStatistically significant; χ2 (categorical) two-sided; P value <0.05; DM: Diabetes Mellitus; LTBI: latent tuberculosis infection.
Effect of disease history characteristics on LTBI in DM patients: In the studied population, 7.49% (35/467) had a family history of TB, 27.62% (129/467) had a history of Bacillus Calmette- Guérin (BCG) vaccination, 4.93% (23/467) had contact with TB patients, 62.10% (290/467) had regular physical examinations and chest X-ray tests, 161 6.21% (29/467) had recent sputum TB test, 3.85% (18/467) had TST within 1 year, 4.71% (22/467) had TB IGRAs test within 1 year, 99.79% (466/467) were not using immunosuppressants. The time to diagnosis of DM in the study population was predominantly 3-10 years (32.98%, 154/467), and DM patients are predominantly adherent to their medications (77.94%, 364/467). LTBI prevalence was not statistically associated with any of these characteristics (P>0.05) shown in Table 4.
| Variable | IGRA+ (n=189,40.47%) N(%) | IGRA- (n=278,59.53%) N(%) | Total (n=467,100%) N(%) | χ2 | P value |
|---|---|---|---|---|---|
| Time to diagnosis of DM (years) | 3.452 | 0.327 | |||
| <1 | 31 (33.7) | 61 (66.30) | 92 (19.70) | ||
| 1~3 | 38 (40.86) | 55 (59.14) | 93 (19.91) | ||
| 3~10 | 70 (45.45) | 84 (54.55) | 154 (32.98) | ||
| >10 | 50 (39.06) | 78 (60.94) | 128 (27.41) | ||
| Family history of DM | 3.23 | 0.072 | |||
| No | 148 (42.90) | 197 (57.10) | 345 (73.88) | ||
| Yes | 41 (33.61) | 81 (66.39) | 122 (26.12) | ||
| Family history of TB | 3.23 | 0.072 | |||
| No | 148 (42.90) | 197 (57.10) | 345 (73.88) | ||
| Yes | 41 (33.61) | 81 (66.39) | 122 (26.12) | ||
| Family history of TB | 0.003 | 0.953 | |||
| No | 175 (40.51) | 257 (59.49) | 432 (92.51) | ||
| Yes | 14 (40.00) | 21 (60.00) | 35 (7.49) | ||
| History of TB | 5.499 | 0.019a | |||
| No | 178 (39.47) | 273 (60.53) | 451 (96.57) | ||
| Yes | 11 (68.75) | 5 (31.25) | 16 (3.43) | ||
| History of chronic kidney disease, gastric ulcer, gastritis, silicosis | 0.15 | 0.699 | |||
| No | 172 (40.76) | 250 (59.24) | 422 (90.36) | ||
| Yes | 17 (37.78) | 28 (62.22) | 45 (9.64) | ||
| History of BCG vaccination | 1.057 | 0.59 | |||
| Not sure | 92 (38.49) | 147 (61.51) | 239 (51.18) | ||
| No | 44 (44.44) | 55 (55.56) | 99 (21.20) | ||
| Yes | 53 (41.09) | 76 (58.91) | 129 (27.62) | ||
| Contact with TB patients | 2.35 | 0.309 | |||
| Not sure | 63 (43.15) | 83 (56.85) | 146 (31.26) | ||
| No | 114 (38.26) | 184 (61.74) | 298 (63.81) | ||
| Yes | 12 (52.17) | 11 (47.83) | 23 (4.93) | ||
| Recent suspected TB symptoms | 4.483 | 0.034a | |||
| No | 183 (41.69) | 256 (58.31) | 439 (94.00) | ||
| Yes | 6 (21.43) | 22 (78.57) | 28 (6.00) | ||
| Regular physical examination and chest X-ray | 1.127 | 0.569 | |||
| Not sure | 16 (42.11) | 22 (57.89) | 38 (8.14) | ||
| No | 61 (43.88) | 78 (56.12) | 139 (29.76) | ||
| Yes | 112 (38.62) | 178 (61.38) | 290 (62.10) | ||
| Had a recent sputum test for TB | 2.774 | 0.096 | |||
| No | 173 (39.5) | 265 (60.5) | 438 (93.79) | ||
| Yes | 16 (55.17) | 13 (44.83) | 29 (6.21) | ||
| Within 1 year of TST | 0.706 | 0.401 | |||
| No | 180 (40.09) | 269 (59.91) | 449 (96.15) | ||
| Yes | 9 (50.00) | 9 (50.00) | 18 (3.85) | ||
| Within 1 year of IGRA | 0.002 | 0.966 | |||
| No | 180 (40.45) | 265 (59.55) | 445 (95.29) | ||
| Yes | 9 (40.91) | 13 (59.09) | 22 (4.71) | ||
| Use of diabetes medications | 3.09 | 0.378 | |||
| None | 28 (40.58) | 41 (59.42) | 69 (14.78) | ||
| Rarely | 3 (30.00) | 7 (70.00) | 10 (2.14) | ||
| Occasionally | 6 (25.00) | 18 (75.00) | 24 (5.14) | ||
| Consistently | 152 (41.76) | 212 (58.24) | 364 (77.94) | ||
| Use of immunosuppressantsb | 0.405 | ||||
| No | 188 (40.34) | 278 (59.66) | 466 (99.79) | ||
| Yes | 1 (100.00) | 0 (0.00) | 1 (0.21) | ||
| Use of home blood glucose monitor | 0.108 | 0.743 | |||
| No | 66 (41.51) | 93 (58.49) | 159 (34.05) | ||
| Yes | 123 (39.94) | 185 (60.06) | 308 (65.95) | ||
| Regular monthly checkups at the community health center or hospital to pick up medication | 0.336 | 0.562 | |||
| No | 34 (37.78) | 56 (62.22) | 90 (19.27) | ||
| Yes | 155 (41.11) | 222 (58.89) | 377 (80.73) |
Note: aStatistically significant; χ2 (categorical) two-sided; P value <0.05; bFisher's test was used for analysis; DM: Diabetes Mellitus; LTBI: Latent Tuberculosis Infection; TB: Tuberculosis; BCG vaccination: Bacillus Calmette-Guérin vaccination; TST: Tuberculin Skin Test.
Table 4: LTBI status of DM patients stratified by disease history characteristics.
A previous history of TB was indicated by 3.43% (16/467) of the study subjects, whose LTBI prevalence of 68.75% (11/16) was significantly higher than the 39.47% (178/451) prevalence in the DM patients without a history of TB (χ2=5.499, P=0.019); Having recent suspected TB symptoms (chest pain, hemoptysis, fever, cough, cough sputum for more than 2 weeks) was indicated by 6.00% (28/467) of the study subjects, whose LTBI prevalence of 21.43% (6/28) was significantly lower than the 41.69% (183/439) prevalence in the DM patients without a history of TB (χ2=4.483, P=0.034) shown in Table 4.
Effect of HbA1c levels on LTBI in DM patients: Among the 264 DM patients who underwent HbA1c testing, HbA1c values ranged from 4.70 to 15.70% with a median of 6.44 (IQR, 5.90-7.32). The prevalence of LTBI was higher in DM patients with HbA1c>7% (49.43%, 43/87) than in DM patients with HbA1c ≤7% (40.68%, 178 72/177) but there was no statistical difference (P>0.05) shown in Table 5.
| Variable | IGRA+ (n=115,43.56%) N(%) | IGRA- (n=149,56.44%) N(%) | Total (n=264,100%) N(%) | C2/Z | P value |
|---|---|---|---|---|---|
| HbA1c | |||||
| Median [IQR] | 6.50 (5.90,7.33) | 6.51 (5.89,7.41) | 6.44 (5.90,7.32) | -0.802 | 0.423b |
| ≤7% | 72 (40.68) | 105 (59.32) | 177 (67.05) | 1.815 | 0.178 |
| >7% | 43 (49.43) | 44 (50.57) | 87 (32.95) |
Note: bMann-Whitney U test was used for analysis; DM: Diabetes Mellitus; LTBI: Latent Tuberculosis Infection; HbA1c: Glycosylated Hemoglobin; IQR: Interquartile Range.
Table 5: LTBI status of DM patients stratified by HbA1c level.
Multiple analysis of factors influencing LTBI in DM patients
Multiple logistic regression was performed using values of gender, age, education level, regular alcohol consumption in the past year, sleep duration, whether intake of meat, eggs and milk more than 3 times/week, use of exhaust fans in housing, family history of DM, previous history of TB, recent suspected TB symptoms, and recent sputum TB testing, which is shown in Table 6. Compared to DM patients with upper junior high school education level, those with an education level of junior high school and below had a higher risk of LTBI (OR=1.689, 95% CI:1.111-2.568; P=0.014); the risk of LTBI was 4.264 times higher in DM patients with previous TB history than in DM patients without previous TB history (OR=4.264, 95% CI:1.258-14.447; P=0.020); and having recent suspected TB symptoms was a protective factor for LTBI prevalence in DM patients (OR=0.316, 95% CI:0.118-0.850; P=0.023) shown in Table 6.
| Variable | P value | OR (95%CI ) | |
|---|---|---|---|
| Sex | |||
| Male | 0.05 | 1.551 (0.999, 2.407) | |
| Female | 1 | ||
| Age, year | |||
| ≤ 40 | 0.645 | 0.687 (0.139, 3.392) | |
| 41~60 | 0.496 | 1.419 (0.518, 3.887) | |
| 61~80 | 0.136 | 2.090 (0.792, 5.512) | |
| ≥ 81 | 1 | ||
| Education level | |||
| Junior high school or below | 0.014a | 1.689 (1.111, 2.568) | |
| Junior high school above | 1 | ||
| Whether regular alcohol consumption in the past year | |||
| Yes | 0.322 | 1.451 (0.694, 3.034) | |
| No | 1 | ||
| Sleep time (hours/day) | |||
| ≥8 | 0.143 | 5.903 (0.548, 63.637) | |
| 6~8 | 0.191 | 4.756 (0.460, 49.209) | |
| 4~6 | 0.243 | 4.094 (0.384, 43.608) | |
| Sleep deprivation/poor sleep quality | 1 | ||
| Regular intake of meat, eggs and dairy products (>3times/week) | |||
| Yes | 0.07 | 1.605 (0.962, 2.676) | |
| No | 1 | ||
| Exhaust fan use in housing (hours/day) | |||
| <6 | 0.767 | 1.133 (0.497, 2.582) | |
| 6~12 | 0.527 | 0.751 (0.310, 1.821) | |
| ≥ 12 | 1 | ||
| DM family history | |||
| Yes | 0.309 | 0.782 (0.487, 1.256) | |
| No | 1 | ||
| History of TB | 0.020a | 4.264 (1.258, 14.447) | |
| Yes | 1 | ||
| No | |||
| Recent suspected TB symptoms | |||
| Yes | 0.023a | 0.316 (0.118, 0.850) | |
| No | 1 | ||
| Recent sputum TB testing | |||
| Yes | 0.191 | 1.715 (0.764, 3.846) | |
| No | 1 |
Note: *Only variables with sufficiently complete data and univariate analysis P<0.2 were included in this analysis; aStatistically significant, multifactorial logistic regression analysis P value<0.05; DM: Diabetes Mellitus; LTBI: Latent Tuberculosis Infection; TB: Tuberculosis; OR: Odds Ratio; CI: Confidence Interval.
Table 6: Multiple logistic regression analysis of factors influencing LTBI status in DM patients*(n =467).
Our findings found that the prevalence of LTBI among DM patients in Nanshan District, Shenzhen in 2019-2020 was 40.47%, which was higher than the prevalence of LTBI among the non- diabetic population in Nanshan District, Shenzhen in 2018 (30.17%, 289/958), indicating that DM patients are a high-risk group for LTBI [25]. The main risk factors associated with LTBI in DM patients were related to a low education level, but also a previous history of TB is associated with LTBI.
Hence, LTBI screening and TB intervention for DM patients in the Nanshan District of Shenzhen is paramount, and an appropriate LTBI screening strategy for the DM population in Nanshan District should be developed. Similar to the results of our study, in a cross- sectional study conducted in the USA, the prevalence of LTBI in patients with type 2 DM (T2D) and pre-DM was 43.4% and 39.1%, respectively [13]. Another observational study from Eastern China using IGRA testing found a 26.9% LTBI prevalence in DM patients [26]. However, a hospital-based study from Atlanta using IGRA testing found a 9.2% LTBI prevalence in newly diagnosed T2D patients, which was much lower than our results [15]. The inconsistency in the results of these studies may be contributed to insufficient sample size and inconsistency in the way DM and LTBI were determined, as well as the possibility of confounding by other underlying diseases. It is therefore important to undertake more research to explore the prevalence of LTBI in DM patients.
Our study demonstrated that DM patients with an educational level of the junior high school or below had a higher risk of LTBI compared to DM patients with an educational level of junior high school above (OR=1.689, 95% CI:1.111-2.568; P=0.014). Similarly, Phan Ai Ping indicated that in T2D patients, a higher educational level was associated with a lower LTBI infection rate (OR=0.08, 95% CI=0.01-0.70; P=0.02) [16]. Maneze found that higher levels of education in DM patients were associated with greater self- management skills [27]. Patients with a low educational level may be less aware of self-protection, have a lower level of knowledge about TB prevention and control, are prone to form poor lifestyle behavioral habits, and poor self-management ability may worsen the course of DM and lead to a higher risk of LTBI. Meanwhile, patients with low educational levels work in mostly crowded and poorly sanitized settings, such as construction sites or factories, and have a higher chance of exposure to patients with active TB disease, which may also contribute to their vulnerability to LTBI [28].
Our results found clear support for the positive correlation between previous TB history and LTBI rates in DM patients (OR=4.264, 95% CI:1.258-14.447, P=0.020). This was consistent with the results of previous studies. For example, a community-based population study in Taiwan identified a previous history of TB (OR=2.08; 95% CI=1.19-3.63) as a risk factor for LTBI in DM patients [9]. Consequently, the management of DM patients with a previous history of TB should be strengthened by increasing the frequency of follow-up visits and performing regular chest radiographs, which can help reduce the incidence of TB.
Interestingly, we found that having recent suspected TB symptoms was a protective factor for LTBI in DM patients (OR=0.316 95% CI:0.118-0.850; P=0.023). This finding has not been reported in the existing literature and we will continue to verify it in a later study with a large sample. We hypothesize that people with recent suspected TB symptoms are likely to actively seek medical help and raise awareness of self-protection, and are more likely to be motivated to learn about TB, thereby reducing their exposure to TB bacteria. The results suggest that timely access to medical care and increased knowledge of TB may help reduce the risk of LTBI. Health education related to TB for DM patients should be enhanced to raise awareness of individual protection, rather than waiting for the population to have suspected symptoms before taking the initiative to learn about them.
Our findings demonstrated that the HbA1c levels in DM patients were not significantly associated with an increased risk of LTBI (P>0.05), which may be related to the insufficient sample size. Consistent with our findings, a Malaysian study using TST testing found that HbA1c levels in DM patients were not associated with an increased risk of LTBI (P=0.787) [29]. In contrast, a Mexican study using TST testing found that poorly controlled DM (defined as HbA1c>7%) was associated with a high risk of LTBI in DM patients (aOR=2.52, 95% CI=1.10-8.25; P=0.04) [30]. It has been suggested that the mechanism of the positive association between HbA1c levels and LTBI risk may be that high HbA1c levels attenuate macrophage antigen-presenting capacity, which leads to increased susceptibility of the organism to Mycobacterium tuberculosis [31,32]. If LTBI risk increases with worsening DM control, stratification of LTBI risk by the level of glycemic control may be clinically useful. Therefore, more studies are needed to explore the association between HbA1c and LTBI.
Notably, the advantages of this study are mainly three. First, this study used IGRA to detect LTBI in DM patients, which has excellent sensitivity and specificity. Given the prevalence of BCG vaccination in newborns in China, compared with TST testing, IGRA testing has higher specificity due to its ability to distinguish MTB from BCG vaccination [33,34]. Moreover, this work is the first study on the prevalence of LTBI and the influencing factors associated with the risk of LTBI infection among DM patients in Guangdong province, adding evidence to explore the relationship between DM and LTBI in China and providing a basis for improving the appropriate LTBI screening strategy for DM population. Lastly, this study was conducted in a community-based study based on a larger sample of the DM population, unlike hospital sampling, and the community survey can be broadly generalized to other settings of the DM population.
However, there are limitations to this study. First, given the cross- sectional design of this study, it is difficult to infer causality with certainty. We were unable to distinguish whether participants acquired LTBI before or after developing DM. Whereas LTBI is unlikely to lead to an increased risk of developing DM, it has been reported since the early 20th century that 85% of the occurrence of TB appears to follow the onset of DM, and a dose- response relationship between MTB infection and DM of varying severity has been found [14,35]. Moreover, this study could not realistically assess the relationship between glycemic control and the risk of LTBI since most participants did not have HbA1c data. Furthermore, this study did not set up non-DM patients as a control group to determine whether DM increases the risk of LTBI or to elucidate whether there are differences in the influencing factors of LTBI between the DM population and the non-DM population. In the future, prospective studies can be conducted to investigate whether DM increases the risk of MTB infection and whether there are differences in the influencing factors of LTBI between the DM population and the non-DM population, using non-DM patients as a control group.
On this basis, we concluded that the prevalence of LTBI was high among DM patients in Nanshan District, Shenzhen; the education level of junior high school or below and a previous history of TB were risk factors for LTBI. Notably, this study is a particularly useful exploration of a community-based active TB screening strategy for DM patients. Given the high rate of LTBI in DM patients, screening for LTBI and early intervention in DM patients can help to shift the control of the TB epidemic to the infected patients and provide a theoretical basis for preventive interventions for LTBI in China, which is of great public health value.
This study was reviewed and approved by the Ethics Review Committee of Shenzhen Nanshan District Chronic Disease Control Hospital, project number 1120180017. Written informed consent was obtained from all eligible participants.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work was supported by the Infectious Disease Prevention and Control of the National Science and Technique Major Project [grant number 2018ZX10715004-002]; the Study on the Role of Plasma-Derived Exosomes in the Diagnosis of Tuberculosis Project [grant number NS2021114]; the Natural Science Foundation of the Guangdong Province of China [grant number 2018A030313123]; the Sanming Project of Medicine in Shenzhen [grant number SZSM201603029] and the Key Disciplines of Medicine in Nanshan District. The funding sources did not have a role in study design, in the collection, analysis and interpretation of Data, or in the decision to submit the paper for publication.
Data will be available on request.
Citation: Zhong T, Liu S, Ye Q, Li S, Guo X (2023) A Study of Latent Tuberculosis Infection and Its Influencing Factors in Diabetic Patients in Nanshan District, Shenzhen, China. Infect Dis Preve Med. 11:312.
Received: 31-Mar-2023, Manuscript No. JADPR-23-23456; Editor assigned: 04-Apr-2023, Pre QC No. JADPR-23-23456 (PQ);; Reviewed: 18-Apr-2023, QC No. JADPR-23-23456; Revised: 25-Apr-2023, Manuscript No. JADPR-23-23456 (R); Published: 02-May-2023 , DOI: 10.35841/2329-8731.23.11.312
Copyright: © 2023 Zhong T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.