Journal of Pharmaceutical Care & Health Systems
Open Access
ISSN: 2376-0419
ISSN: 2376-0419
Research Article - (2022)Volume 9, Issue 4
This study was designed to develop a reliable method for estimation of 3TC, TFV, and EFV in pure and its pharmaceutical dosage form by RP-HPLC. Method: A simple, rapid, accurate, precise, robust and isocratic reverse phase high performance liquid chromatographic (RP-HPLC) method was developed for the estimation of 3TC, TFV and EFV in combined dosage form. The method was developed by using Inertsil ODS-3V (250 × 4.6, 5 μm) column, with Mobile phase composition of Acetonitrile: 1% IPA in the ratio of 85:15 at a flow rate of 1 ml/min and the effluents were monitored at 256 nm using PDA detector. Results: 3TC, TFV and EFV drugs were eluted at retention time of 2.4, 2.8 and 4.5 min (± 0.5). The proposed method was validated as per ICH guidelines. The correlation coefficient (R2) was found to be 0.999. All the parameters were found to be within the limits. The recovery studies were carried out and found to be within 98-102% and the %RSD was found to be ˂2%. Limit of Detection and Limit of Quantification of 3TC, TFV and EFV were found to be 0.06, 0.09, 0.17 μg/ml and 0.18, 0.27, 0.53 μg/ml respectively. Conclusion: The Analysis time for the proposed method is less which can be applied for the multiple samples in less time. Hence the proposed method was found to be novel, simple, accurate and robust and the validation studies indicated that the proposed methods are suitable for the routine quality analysis of the combined dosageform.
Lamivudine; Tenofovir; Efavirenz; RP-HPLC; ICH guidelines
The 3TC, 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5- yl]pyrimidin-2- one Figure 1a is a nucleoside analogue and reverse transcriptase inhibitor used in the therapy of Human Immunodeficiency Virus (HIV) and Hepatitis B Virus (HBV) infection. It is phosphorylated to active metabolites that compete for incorporation into viral DNA. They inhibit the HIV reverse transcriptase enzyme competitively and act as a chain terminator of DNA synthesis. The lack of a 3'-OH group in the incorporated nucleoside analogue prevents the formation of the 5' to 3' phosphodiester linkage essential for DNA chain elongation, and therefore, the viral DNA growth is terminated. TFV, {[(2R)-1-(6- amino-9H-purin-9-yl) propan-2-yl] oxy} methanephosphonate Figure 1b is an acyclic analog of deoxyadenosine 5'-monophosphate (d-AMP). That lacks a hydroxyl group in the position corresponding to the 3' carbon of the d-AMP, preventing the formation of the 5 to 3′phosphodiester linkage essential for DNA chain elongation. Inhibitors of reverse transcriptase (RNADIRECTED DNA POLYMERASE), an enzyme that synthesizes DNA on an RNA template, preventing viral replication. EFV, (4R)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-1H-3,1 benzoxazin-2-one.Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI), induces activity of the cytochrome P450 system directly binds to the HIV-1 RT and RNA dependent DNA polymerase, blocking its function in viral DNA replication. It leads to reduce HIV viral load, retarding damage to the immune system and reduce the risk of developing AIDS. Literature review reveals that analytical methods based on RP-HPLC, dissolution studies by RPHPLC methods for the determination of 3TC, TFV and EFV [1].
However, in most of the methods, mobile phase used consists of Buffer and there is no method reported regarding the use of IPA for the determination of 3TC, TFV and EFV (Figure 1c).
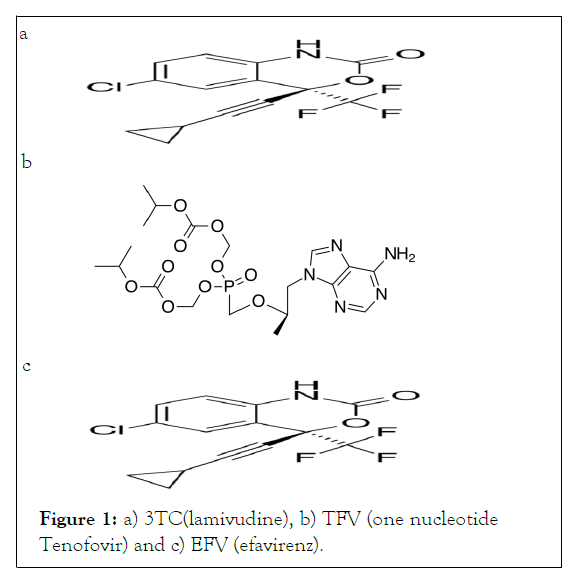
Figure 1: a) 3TC(lamivudine), b) TFV (one nucleotideTenofovir) and c) EFV (efavirenz).
Determination of solubility of drugs
Drugs were dissolved in different solvents and solubility was checked. Hence ACN and 1% IPA in the ratio of 85:15 were used as a solvent for solubility of drug.
Selection of detector wavelength
A standard solution of 3TC, TFV and EFV of concentration 100μg/ml was prepared and scanned in the UV region i.e.; 200 to 400 nm using photodiode array detector to detect the maximum wavelength. The spectrum was recorded and its isobestic point showed at 256 nm [2].
Preparation of 3TC, TFV and EFV standard solution
Weighed separately and transferred accurately 10 mg of 3TC, TFV and EFV standard drug into a 10 ml volumetric flask. Then added about 4 ml of diluent and solicited it to dissolve completely and makeup to the mark with the same solvent to 10 ml (1000 μg/ml).
Preparation of working standard solution
Pipetted out 0.1 ml of 3TC, TFV and EFV from primary standard solution into a 10 ml volumetric flask. Then added about 4 ml of diluent and sonicated it to dissolve completely and makeup to the mark with the same solvent to 10 ml (10 μg/ ml).
Preparation of sample solution
The average weight of tablets were determined by weighing 20 tablets and powdered. 1.7 g of equivalent weight of tablet power was weighed and transferred into 10 ml volumetric flask and about 4 ml of diluent was added and degassed for 15 minutes for the complete dissolution of drug, volume was made up to the mark with diluent and mixed. Above solution was filtered through what’s man filter paper i.e.; primary stock. From the above solution 1 ml was pipetted out and transferred into 10 ml volumetric flask and made up to the mark with diluent. This solution was used for assay studies.
Optimized chromatographic conditions
The HPLC system (Shimadzu, spd-M20 A) equipped with Inspire, C18, (4.6 × 250 mm, 5 μm) column component having temperature control, PDA detector and Rheodyne injector control, PDA detector and Rheodyne injector. The Mobile phase consists of Acetonitrile: 1% IPA in the ratio of 85:15. The mobile phase was pumped with 1.0 ml/min flow rate from the solvent reservoir to the column. The injection volume was 20 μl. The column oven temperature was maintained at 300°C. The eluents were detected at 256 nm in Figure 2.
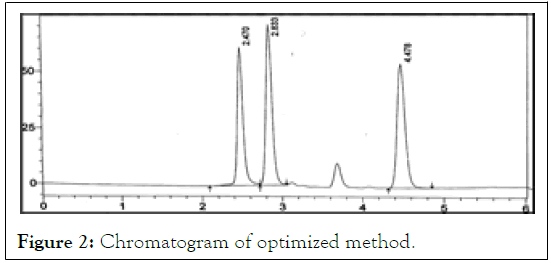
Figure 2: Chromatogram of optimized method.
Method validation
Linearity: From the primary stock solution pipetted out 0.04,0.08,0.12,0.16,0.2 ml of 3TC; 0.1,0.2,0.3,0.4,0.5 ml TFV and 0.08,0.16,0.24,0.32,0.4 ml of EFV solution and transferred into 10 ml volumetric flask and made up to the mark with diluent to obtain the concentrations 4,8,12,16,20 μg/ml of 3TC; 10,20,30,40,50 μg/ml of TFV and 8,16,24,32,40 μg/ml EFV. The solutions were degassed and passed through 0.45 μm membrane filter for filtration. The concentrated solutions of 3TC, TFV and EFV were prepared and injected. A calibration curve was produced by analyzing correlation coefficient was determined by regression analysis [3].
Acceptance criteria: Square of correlation coefficient should not be less than 0.999.
Precision
From the primary stock solution of 3TC, TFV and EFV pipetted out 0.12 ml, 0.3 ml and 0.24 ml of solution and transferred into six 10 ml volumetric flasks and make up the volume with the diluents to obtain the concentrations of 12 μg/ml of 3TC, 30 μg/ml of TFV and 24 μg/ml of EFV [4].
The solution was degassed and passed through 0.45 μm membrane filter for filtration. This solution was injected for six times into HPLC in the same day and the area for six replicate injections should be within the specified limits, and the chromatograms were recorded. Acceptance criteria: %RSD for six standard injections should not be more than 2%.
Intermediate precision
From the primary stock solution of 3TC, TFV and EFV pipetted out 0.12 ml, 0.3 ml and 0.24 ml of solution and transferred into six 10 ml volumetric flasks and make up the volume with the diluents to obtain the concentrations of 12 μg/ml of 3TC, 30 μg/ml of TFV and 24 μg/ml of EFV. The solution was degassed and passed through 0.45 μm membrane filter for filtration. This solution was injected for six times into HPLC on next day and the area for six replicate injections should be within the specified limits, and the chromatograms were recorded [5].
Acceptance criteria: %RSD for six standard injections should not be more than 2%.
Accuracy
Accuracy was performed in 3 different levels for 3TC, TFV and EFV spiked known quantity of these 3 drugs yet 50%, 100%, 150% level into the analyzed samples in triplicate for each level. From the results %recovery was calculated. 3TC, TFV and EFV reference standards were accurately weighed and added to a sample mixture, at three different concentration levels (18 μg/mL, 24 μg/mL, 30 μg/mL of 3TC, 45 μg/mL, 60 μg/mL, 75 μg/mL of TFV and 36 μg/mL, 48 μg/mL, 60 μg/mL of efevirenz). An each level, samples were prepared in triplicate and the recovery percentage was determined.
Robustness
Sample solution were prepared and analyzed under the established conditions and by variation of the following analytical parameters flow rate of Mobile phase (0.8 ml/min, 1.2 ml/min), column temperature (25°C, 35°C) and detection wavelength (251 nm, 261 nm). Ivacaftor and Tezacaftor were determined for each condition and the obtained data were submitted for statistical analysis and the calculated results were shown in Table 1.
| S.NO | Parameters | Normal | Variation | %Recovery | ||
|---|---|---|---|---|---|---|
| 3TC | TFV | EFV | ||||
| 1 | Wavelength | 256 nm | 251 nm | 99.3 | 84.5 | 155.6 |
| 261 nm | 105.8 | 99.6 | 40.8 | |||
| 2 | Temperature | 30ºC | 25ºC | 103 | 100.2 | 102.6 |
| 35ºC | 102 | 100.2 | 102.2 | |||
| 3 | Flow rate | 1 ml/min | 0.8 ml/min | 125 | 121.5 | 126.1 |
| 1.2 ml/min | 84.7 | 83 | 84.1 | |||
Assay
The average weight of tablets were determined by weighing 20 tablets and powdered. 1.7 g of equivalent weight of tablet power was weighed and transferred into 10 ml volumetric flask and about 4 ml of diluent was added and degassed for 15 minutes for the complete dissolution of drug, volume was made up to the mark with diluent and mixed. Above solution was filtered through what’s man filter paper i.e.; primary stock. From the above solution 1 ml was pipetted out and transferred into 10 ml volumetric flask and made up to the mark with diluent and injected shown in Figure 3.
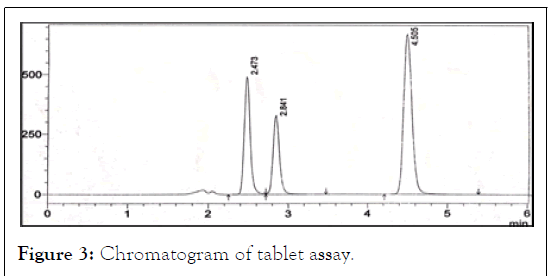
Figure 3: Chromatogram of tablet assay.
Chromatographic parameters were optimized to develop a HPLC method for the simultaneous estimation of 3TC, TFV and EFV, short analysis time (<5min) and acceptable resolution (Rs>2) various composition of Mobile phase like; ACN:1% IPA (60:40 v/v), ACN:1%IPA(70:30v/v), ACN:1% IPA(80:20), ACN:1%IPA(90:10), were tried at flow rate of 1 ml/min. Acetonitrile and 1%IPA in ratio of 85:15 showed symmetrical peaks with good resolution. The optimum Wavelength for detection was 256nm and the RT was found to be 2.47, 2.833 and 4.478 mins respectively. Linearity curve was obtained in the concentration range of 4-20, 10-50 and 8-40 μg/ml. LOD and LOQ were determined from the slope and SD of Y-intercept of regression line of the calibration curve. The precision of the method, instrument precision were evaluated and the %R.S.D values were less than 2%. The accuracy of the method was determined by recovery studies. The recovery studies were close to 100% which complies with ICH Guidelines. Developed method was found to robust while changing the flow rate wavelength detection, temperature. The proposed method is superior when compared to the reported method with less RT and Good separation and can be applied for quality control of drugs in pharmaceuticals [6].
An effort has been made to develop a simple, accurate method to estimate 3TC, TFV and EFV in bulk and pharmaceutical preparation and to validate the method, according to ICH guidelines by using Inertsil ODS-3V (250 × 4.6, 5 μm) column, with Mobile phase composition of Acetonitrile: 1% IPA in the ratio of 85:15 at a flow rate of 1 ml/min and the effluents were monitored at 256 nm using PDA detector. 3TC, TFV and EFV drugs were eluted at retention time of 2.4, 2.8 and 4.5 min (± 0.5). The proposed method was validated as per ICH guidelines. The correlation coefficient (R2) was found to be 0.999. All the parameters were found to be within the limits. The recovery studies were carried out and found to be within 98-102% and the %RSD was found to be 2%. Limit of Detection and Limit of Quantification of 3TC, TFV and EFV were found to be 0.06, 0.09, 0.17 μg/ml and 0.18, 0.27, 0.53 μg/ml respectively. The results obtained are in good agreement and can be used for the routine Analysis of Ivacaftor and Tezacaftor in combined dosage form in laboratories and for Quality control purpose [7-8].
The present method was developed and validated using 1% IPA: ACN. IPA enhances the selectivity of binary or tertiary mixtures and it is a transition eluent lying between aqueous and nonaqueous eluents. It is always used with the aqueous mobile phase as it acts as a co solvent in the mixture. The main reason for the use of IPA as a part of aqueous mobile phase is to keep the column free of undesired adsorbed contaminants from sample or a sample matrix. As this may change the retention behaviour of analytes or as a baseline noise or drift. By using IPA which acts as a good washing agent of the column when compared to other solvents. Iso proponal can lead to reduced diffusion rates resulting in peak broadening as well as creating excessive high back pressure in column. Hence organic phase or modifier in high concentration can overcome this problem. The proposed method was found to be simple, precise, and accurate; isocratic with low %RSD’s and was able to estimate the analytes at lower linearity ranges when compared to other existing methods.
I would like to express our sincere thanks to the management and Principal Dr P. Raviprakash of Creative Educational Society’s college of Pharmacy for the facilities, requirements and constant support during the research work.
The authors declare that they have no competing interests.
Citation: Rao N (2022) A New Validated Rp-HPLC Method Development for the Simultaneous Estimation of Lamivudine, Tenofovir and Efavirenz in Pure and its Pharmaceutical Dosage Form. J Pharma Care Health Sys. 9:251.
Received: 08-Jul-2022, Manuscript No. JPCHS-22-17270; Editor assigned: 13-Jul-2022, Pre QC No. JPCHS-22-17270 (PQ); Reviewed: 27-Jul-2022, QC No. JPCHS-22-17270; Revised: 02-Aug-2022, Manuscript No. JPCHS-22-17270 (R); Published: 09-Aug-2022 , DOI: 10.35248/2161-0983.22.9.251
Copyright: © 2022 Rao N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.