
Journal of Perioperative & Critical Intensive Care Nursing
Open Access
ISSN: 2471-9870

ISSN: 2471-9870
Review - (2019)Volume 5, Issue 1
Leukemia is a group of blood cancers that influences the blood and bone marrow. Notwithstanding efficient treatment, causes of leukemia stay puzzling. There are many potential environmental hazards must be understood, in view of the fact that most requiring a biological justification or logical epidemiological evidence. Many influences might strengthen the possibilities of developing leukemia. These involve a specific genetic predisposition, radioactive rays, X-rays and certain chemical substances. Likewise, viruses consider playing a role in an infrequent pattern of leukemia. Another risk is cigarette smoking. No triggering factors can be specified for a wide class of leukemia’s.
Leukemia; Myeloproliferative disorders; Etiology
Down Syndrome Disorder (DS); Acute Lymphoblastic Leukemia (ALL); B-cell Precursor (BCP); Myeloid Leukemia in Down Syndrome Disorder (ML-DS); Acute Myelomonocytic Leukemia (AML); Occupational Safety and Health Administration (OSHA); Part Per Million (ppm);: Chronic Myeloid Leukemia (CML); Human T-lymphotropic Virus (HTLV)
Our intentions with this review are to reach climax the new advances and offer a mindset on the ongoing knowledge of the etiology of leukemia rather than being a pure-comprehensive assessment.
Leukemia is cancer of the tissues that produce blood in the body, including the bone marrow and the lymphatic system. There are many types of leukemia. Some forms of leukemia are more usual for children others take place in adults [1]. Childhood leukemia is a group of disorders with diverse immunophenotypes and genetic transformations. The realization of causes and prevention/early intervention is doubtless a valuable target [2]. Recognized causes include ionizing radiation and congenital genetic syndromes such as Down’s, neurofibromatosis, Fanconi’s anemia, and Bloom’s Syndrome, all of which explain less than 10% of cases. The prevalence of the disease has raised close to 1% per year in the later two decades [3] with comparable ratios of increase decades earlier [4,5], noticing that causals influence the disease are expected to have come into a more prevalent in the former’s population. In the last decades, epidemiologic and genetic researches have given evidence suggesting ionizing radiation and chromosomal abnormalities in the genesis of leukemia. The epidemiologic evidence for the viral etiology of human leukemia is insufficient despite the hope driven by continuing successes in detecting leukemogenic viruses in mice. Epidemiology may specify a disease, seek it or test specific suggestions. The leukemia fatality that has been determined by descriptive epidemiology, still they do not per suggest the etiology [6]. The uniform definition of childhood leukemias under current scientific protocols gives suitable subgroup information about etiologic studies and will become a significant cause of leukemia epidemiology studies. Following studies would progress from the earlier description of cancer clusters of active control at cancer reports, the capture of complete residential histories for cancer cases and prospective population-level archiving of biological data to enable proper environmental and viral testing.
A more critical look at the etiology of leukemia
Leukemia occurrence is omnipresent within the world. Studies have looked for the cause of leukemia since The 1940s. The investigators cited several types of causes on this cancer. Although, dramatic biologic advances have expanded understanding of leukemogenesis. There is still a great uncertainty on the causes that develop leukemia. In most patients, leukemia develops because of accidental errors during the blood cells formation. There are multiple hypotheses of the reason behind leukemia. No single acknowledged cause for various leukemias exists. Debatable hypotheses was planned to suggest the role of physical factors (i.e., high dose ionizing radiation, electromagnetic fields), and chemical factors (i.e. benzene, formaldehyde), genetical (endogenous) factors (i.e., Down’s syndrome, mutations in bone marrow), familial (hereditary) factors (i.e., Fanconi anemia), lifestyle factors (i.e. smoking, obesity, dietary intake), biological factors (i.e., adrenal hormones, animal sources), autoimmune disorders’factors (i.e., rheumatoid arthritis, autoimmune haemolytic anemia, ulcerative colitis), chemotherapeutics factors, (i.e., Chlorambucil, Melphalan, Cyclophosphamide), blood disorders’ factors (i.e., Myelodysplastic syndrome and Myeloproliferative disorders) and even infectious factors (i.e., viruses) as being answerable for leukemia incidents. The real cause of leukemia is continues to be underneath scrutiny. Through this review, we tend to aim at supplying an updated insight of the pathological process and to provide clues for potential etiology of leukemia (Figure 1)
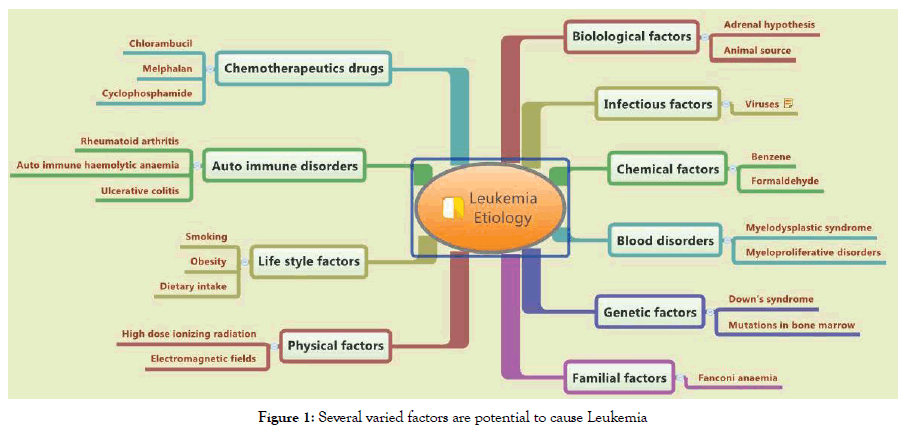
Figure 1. Several varied factors are potential to cause Leukemia
Search strategy
We used PubMed, Embase, and Web of Science to look through the literature. Inquiry terms included leukemia etiology and hereditary variables; a similar arrangement of the terms was adopted for all databases related to the headings that were custom-made for every database. The content word seeks determined leukemia etiology in the title, a hereditary term in the title or conceptual and humanrelated terms in the title or abstract. This review incorporated casecontrol studies that examined a least of one. We excluded focuses on non-human (e.g., animal leukemia etiology).
Genetical (endogenous) factors
Are the genetic defects typical of leukemia hereditary?
Leukemias are not hereditary in the true sense. However, it has been found that the risk of developing this cancer is increased when malignant diseases have occurred often in the family. This suggests that a specific predisposition may play a role in the development of leukemia. Specific genetic conditions may increase the risk of leukemia. A part of is the so-called Down syndrome, caused by an innate genetic change.
Down’s syndrome: For most leukemic sufferers, there’s no familial defect of the genetic material. Children with Down syndrome disorder (DS) have an increased risk of promoting acute lymphoblastic leukemia (ALL) characterized by an additional heterogeneous pattern of hereditary findings. These highlights feature the role of Down syndrome in leukemogenesis [7]. They have an expanded risk of B-cell precursor (BCP) acute lymphoblastic leukemia (ALL) [8]. Myeloid leukemia in down disorder (ML-DS) is observed as retrogradation with distinct clinical and organic features. There are few studies focusing on the clonal cytogenetic differences amongst the progress of ML-DS. The molecular cytogenetic examinations performed, permitted the description of novel chromosomal irregularities in ML-DS and potential candidate genes concerned within the leukemogenic process [9]. On the opposite hand, Amorim et al. have conducted a study to find out whether 677C-->T and/or 1298A-->C polymorphisms of MTHFR gene would play an extra role within the status of acute myelocytic leukemia (AML) in DS youngsters [7]. The study revealed a none association between these polymorphisms and so the risk of AML in DS youngsters. The data likewise prove that it does not relate MTHFR polymorphisms to the chance of being a DS child [10].
Familial (hereditary) factors: Leukemia has long been observed to occur more among members of certain families than in the average population. The prime evidence on the genetic processes controlling familial leukemia emerged in 1990 when Li-Fraumeni syndrome was associated with TP53 mutations. Since this discovery, experts have determined many genes linked to a hereditary predisposition to leukemia [11]. Stjernfelt et al. examined and compared demographics in families with one case of childhood cancer to families with multiple cases of childhood cancer [12]. There is a high proportion of children with leukemia having a childhood relative of the same diagnosis suggests a hereditary background. Tawana et al. reported a new RUNX1 family in three sisters holding a germline nonsense RUNX1 variant, they showed signs of acute myelomonocytic leukemia (AML) at 5 years of age. Investigation of entire exome sequencing of tumor samples revealed that the three siblings independently acquired myeloproliferative neoplasms [13]. The clinical and molecular homogeneity over these three young siblings provides the principal prominent example of convergent AML evolution in a RUNX1 pedigree giving rise to high-risk AML [13]. Archer et al. have conducted an exome-wide association study of childhood (ALL) among Hispanics to affirm and recognize novel variants associated with disease risk in this ethnic group [14]. A case-parent trio study design was used to avoid issues with population stratification bias among this at-risk ethnic group. The assessment uncovered none potent novel hereditary genetic risks of (ALL) among this ethnic group.
Lifestyle factors (i.e., smoking, obesity)
Several inquiries left: For this purpose, researchers have carried many studies, for example, on the effects of chemicals or food ingredients. Likewise, great groups of individuals have been asked about their habits and potential influencing factors. A connection between leukemia and the personal lifestyle, overweight, is still under discussion. But, experts do not verify these interactions.
Obesity: Obesity is related to an increased incidence of the many cancers, as well as leukemia, though it's unknown whether malignant neoplastic diseases incidence is augmented directly by fatness [15]. Animal models were developed to test whether obesity is correlated with acute lymphoblastic leukemia (ALL). Thus, the determined associations between fatness and leukemia incidence are possible to be directly associated with biological effects of obesity [15]. Other research works offer many evidences that the association with obesity is a risk issue for hematologic malignancy [16-18].
Smoking: The results of earlier researches on the influence of smoking on the risk of leukemia are not evident. However, evidence that smoking increases the risk of developing acute myeloid leukemia. Several studies have been directed in leukemic smoker’s subjects to identify the association of smoking with leukemia. The scientists look at the chance that smoking cigarettes can increase the risk of developing acute myeloid leukemia. Since cigarette smoke (tobacco) has benzene and radioactive matter that may induce leukemia [19]. It is known cigarette smoking induces leukocytosis and enhanced genetic instability in normal healthy individuals [20]. Many epidemiological studies have conducted in smokers patients provides no proof that smoking bears any major relationship to the incidence of leukemia [21,22]. Other studies showed it was potential that fag smoke might act as a promoter or co-carcinogen within the transformation of chronic myelogenous leukemia [20,23]. There are many studies that explored interactions between antepartum exposure to maternal smoking and polymorphisms in metabolic genes within the risk of childhood cancer of the blood (ALL). Assuming, smoke smoking habits are the activating agent of antepartum malignant neoplastic diseases. The studies did not prove the interaction between CYP1A1*2A/2B and maternal smoking. The association with NAT2*5 and so the gene-gene interactions ought to be duplicated [19,24].
Biological factors
Adrenal hormones: The corticosteroid has a unique role in the fetus in inducing a wide range of enzymes before birth on which survival after birth is dependent. Recently, a replacement hypothesis was planned for the pathological process of childhood acute lymphocytic leukemia (ALL). The supposed ‘adrenal hypothesis’ emphasized the role of endogenous hydrocortisone within the etiology of B-cell precursor ALL [25-27]. The pattern of infections in the initial years of life modulates our immune system, and a low incidence of infections has been coupled to an increased risk of common childhood acute lymphoblastic leukemia (ALL) [25,27]. Viral infections increase corticosteroid levels through activation of the hypothalamic-pituitary-adrenal (HPA) axis by cytokines [28]. Interpretation of the adrenal hypothesis proposes that the chance of childhood ALL is reduced once infancy infections induce qualitative and quantitative changes within the hypothalamuspituitary- adrenal axis that increases plasma corticoid levels. This may eliminate leukemic cells even preleukemic cells originating during fetal life for the (ALL) subsets that dominate within the first 5-7 years of life [25,27] and should moreover suppress the Th1- dominated pro-inflammatory response to infections, and so lower the proliferative stress on pre-existing preleukemic cells [27].
Chemical factors
Benzene: Benzene was employed as a solvent, and these chemicals were considered unsafe. Leukemia induced by the proven professional handling of benzene as an occupational disease. Benzene is a leukemogenic, and thus the Occupational Safety and Health Administration (OSHA) is trying to limit exposure to it. The projected new regulation could be a limit of an eight-hour timeweighted average of one part per million (ppm) in situ of this limit of ten ppm [29]. Benzene has the miserable privilege of being the sole industrial chemical leading to chronic conditions in vulnerable people exposed to its gases. Hence, benzene has been inserted within the list of human carcinogens. There’s still no agreement of the role benzene in chronic varieties of cancer of the blood [30]. A case reports on occupational intoxication, with moderate anemia and hypogranulocytosis, within the course of that happens hairy cell leukemia, benzene considered as a cytotoxic etiology of this leukemia [31]. Costantini et al. directed a population-based casecontrol study to find the association between exposure to organic solvents and risk of myeloid and lymphoid leukemia. Consultants assessed exposure at the individual level to a variety of chemicals. There have been elevated point estimates for the associations between medium/high benzene exposure and chronic lymphatic leukemia. Benzene elevated risks of chronic lymphatic leukemia even though with wide confidence intervals [32].
Physical factors
Radioactive rays and X-rays: The influence of ionizing radiation, a collective term of X-ray, ultraviolet and radioactive radiation, has long been suspected as a possible cause of leukemia. Before this risk was recognized, radiologists who were exposed to high doses of radiation for a prolonged period became much more likely to develop leukemia than would be expected from the normal chance risk. Radioactive rays, as shown by extremely high radiation exposure, as caused by the nuclear bomb blasts in Japan during the Second World War and the reactor shortfall in Chernobyl and Fukushima, are now considered to be a confirmed risk factor of leukemia. This correlation was most convincingly established in a study of the survival of residents in Hiroshima and Nagasaki after the atomic bombardment in 1945, which clearly demonstrates an increased risk of the development of acute myeloid leukemia (AML) and chronic myeloid leukemia (CML) [33]. The high-energy radiation produces damage in the genome of body blood cells that often divide. These include the cells of the bone marrow. The damage of blood-forming cells can lead to the development of leukemia. The higher the radiation dose exposure to the person, meaning the higher the disease risk [34]. Using X-rays for examination are considered as causing leukemia. Therefore, X-ray examinations should only be performed when they are really necessary. By documenting X-ray examinations in special “X-ray passes” and storing X-ray images, patients can help to avoid unnecessary exposures [35].
Infectious factors (i.e., viruses)
Viruses: There are three existing hypotheses on infectious mechanisms in the etiology of childhood leukemia:
1. Exposure to the uterus or around the time of birth.
2. Delayed exposure to frequent infections beyond the early year of life.
3. Unusual population mixing.
No specific virus has been identified with childhood leukemia and no evidence up to now of viral genomic inclusions within leukemic cells. The case-control and cohort studies have disclosed ambiguous results. There is unsatisfied evidence from studies on early childhood infectious exposures, vaccination, and social mixing [36]. Childhood leukemia is the major subtype of pediatric cancer and, despite success in treatment, its causes remain mysterious. Although there may not be one and solely cause, an abnormal immune response to common infection(s) has emerged as a reasonable etiological mechanism. A rise of candidates environmental exposures had been proposed, but most lack a biological rationale or compatible epidemiological evidence [37]. Reports on British patients that have been followed longitudinally (from 1947 to 2000) have suggested that time trends may be associated with the etiology. The results are in keeping with their hypotheses that, some childhood leukemia could also be triggered by infection occurring near the time of diagnosing as leukemia, notably in conditions of low herd immunity, and lift the chance that contact with influenza shortly before the diagnosing of leukemia could occasionally be concerned [38]. Viruses are urged to play a role in the pathological process of ALL. Transforming viruses could integrate into the genome of precursor B-cells, disturbing differentiation and proliferation management. Viruses could promote leukemogenesis indirectly by eliciting an abnormal immunologic response leading to autonomous B-cell precursor proliferation [39]. Recent experimental support for the hypothesis of “delayed infection” as a cause of childhood leukemia proposed by Mel Greaves originated from Martin-Lorenzo et al. and Swaminathan et al. who proved that exposure of genetically susceptible mice to infection can cause leukemia. The examinations of ALL cases suggest that the sought-after agent could be a common virus or other infectious agents (e.g., transforming bacteria or unicellular organisms) [40].
Human T-lymphotropic (HTLV) viruses cause a rare T cell leukemia: Human T-lymphotropic virus (HTLV), found mainly in Japan, the Caribbean, and certain parts of Africa and South America, affects human T cells and, in rare cases, can cause a particular form of T cell leukemia. The risk of suffering from HTLV in this particular leukemia is 1-2%. For other human leukemias, still insufficient evidence that viruses or other pathogens are involved in the disease. These leukemias are considered non-contagious or transferable [41].
The continued scientific information shows that viral infection plays a probable role within the development of ALL, maybe in association with different risk factors. Future studies ought to adopt the applying of advanced microbial detection assays for viral agents. We want well-designed investigations of enough sample size to link the previous infection with subsequent leukemia. We also need extensive prospective cohort studies that follow children, and store serial samples from birth — probably alongside with maternal pregnancy samples — would possibly retrospectively or prospectively be able to correlate past or present infections with leukemia. Such studies might give insight into whether early infectious exposures are needed to promote a normally functioning immune system and to decrease abnormal lymphoproliferative responses.
Not applicable.
Mohammed E.H Ournasseir, Najem Aldin M. Osman and Mahjoob O. Mahjoob wrote and revised this article. The authors read and approved the final manuscript.
We thank Prof. Ali S. Elwakeel at the Department of Microbiology at Omdurman Islamic University, Faculty of Medical Laboratory Sciences for the critical proof-reading of this manuscript.
The authors declare that they have no competing interests.
No funding for this research.
Citation: Elawad HE, Ournasseir MEH, Osman NAM, Mahjoob MO (2019) A Monograph on Clues to the Etiology of Leukemia. J Perioper Crit Intensive Care Nurs 5:146. 10.35248/2471-9870.19.5.146.
Received: 21-Feb-2019 Accepted: 26-Apr-2019 Published: 03-May-2019
Copyright: © 2019 Elawad HE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.