Endocrinology & Metabolic Syndrome
Open Access
ISSN: 2161-1017
ISSN: 2161-1017
Research Letter - (2014) Volume 3, Issue 4
Background: Depression is a common comorbidity among patients with type 2 diabetes. The emotional consequences of diabetes have been scrutinized by a number of investigative teams and there were varying reports about the association of depression and type 2 diabetes. However, there is limited data about this in Ethiopia.
Objective: To assess the associated risk factors of co-morbid depression among type 2 diabetic out patients presenting to Black Lion General Specialized Hospital, Addis Ababa, Ethiopia.
Method: A cross-sectional study was conducted on a random sample of 276 type 2 diabetic outpatients from Black Lion General Specialized Hospital. Systematic random sampling technique was used to get each individual respondents. Data regarding patient characteristics were collected using structured interviewer administered questionnaire and health related information were collected from patient chart. Association between depression and type 2 diabetes among patients were explored using multiple logistic regression model.
Result: Totally 264 study participants were interviewed with a response rate of 95.6%. Of whom those interviewed participants 53.0% were female, 69.3% were married, 80.7% were Orthodox Christian, and 57.2% were Amhara. The mean + standard deviation age at diagnosis and current age of the subjects were 43.9 + 10.9 and 55.9 + 10.9 years respectively. The statistically significant risk factors was poor social support (odds ratio 14.7, 95% CI 1.94-111.89, p-value 0.01).
Conclusion: This study demonstrated that depression is a common co-morbid health problem in type 2 diabetic out-patients. Poor social support was the risk factor significantly associated with depression among type 2 diabetes patients. In a setting where recognition, screening and treatment levels remain low, health care providers need to focus their efforts on diagnosing, referring and effectively treating co-morbid depression in order to deliver rights-based and client-centered services for people in real needs.
Keywords: Diabetes; Depression; Adult patient; Co-morbidity; Associated factors; Outpatient; Ethiopia; Cross-sectional study
AAU: Addis Ababa University; BMI: Body Mass Index; CAD: Coronary Artery Diseases; CI: Confidence Interval; CSA: Central Statistical Agency; DSM: Diagnostic Statistical Manual of Mental Disorders; EDID: European Depression in Diabetes; E.g.: Example; et al.: et alia; EFY: Ethiopian Fiscal year; ETB: Ethiopian birr; FBS: Fasting Blood Sugar; FDRE: Federal Democratic Republic of Ethiopia; FMOH: Federal Ministry of Health; HbA1c: Glycosylated hemoglobin; IBM: International Business Machine; INR: Indian Rupee; Kg/m2: Kilogram per meter square; MDD: Major Depressive Disorder; MOH: Ministry of Health; NGOs: Non-Governmental Organizations; OR: Odds Ratio; PHQ: Patient Health Questionnaire; PVD: Periphero Vascular Diseases; SPSS: Statistical package for Social Science; Sq km: Square Kilometer; T2DM: Type 2 Diabetes mellitus; UK: United Kingdom; USA: United States of America; Vs: Versus
Diabetes mellitus is a chronic metabolic disease, characterized by a disorder in the metabolism of carbohydrates, lipids and amino acids, either as a result of decreased insulin secretion , or due to a reduction to insulin sensitivity of the cells of the body cells. It is a disease that acquires epidemic form and constitutes one of the major threats to human health in 21st century [1]. The World Health Organization projected that 300 million people will suffer from diabetes by 2025 [2]. Diabetes, the fourth leading cause of death has affected an estimated 246 million people in the world [3]. Type 2 diabetes accounts for 90 to 95% of the incidence of diabetes and is associated with a strong genetic predisposition as well as age, obesity and lack of physical activity [4].
The prevalence of diabetes is growing significantly throughout the world, and Sub-Saharan Africa is no exception. In 2010, 12.1 million people were estimated to be living with diabetes in Africa, and this is projected to increase to 23.9 million by 2030 [5].
Ethiopia, which is one of the developing nations, is at a risk of increased diabetes incidence. In 2012 the estimated diagnosed diabetes cases (20-79 years) and estimated numbers of people with undiagnosed diabetes (20-79 years) in 1000s in Ethiopia is 1,386.64 and 1,145.50 respectively. In addition, the estimated diabetes-related death is 23,869 (20-79 years) [6].
Depression is a serious chronic disease that is associated with more functional disability than many other chronic diseases. Depression is the fourth most important contributor to the global burden of disease and in year 2000 comprises 4.4% of the total Disability Adjusted Life Years [7]. Previous work on the relationship of diabetes and depression has often focused on major depressive disorder; however, subsyndromal depressive conditions are far more prevalent than major depressive disorder and are linked to increased disability, risk of health decline, and health care use, and premature mortality [8].
The emotional consequences of diabetes have been scrutinized by a number of investigative teams [9]. A bidirectional association has been found between depression and diabetes mellitus [10]. Type 2 diabetes and depression are two long course diseases with modifiable risk factors. Among patients with type 2 diabetes, depression is found to be strongly associated with increased morbidity and mortality [2,3,11- 13]. Similarly, depression is a common and costly comorbidity among people with diabetes [14,15]. Likewise surveys and meta–analyses conducted on diabetes mellitus and depression have shown that the existence of diabetes mellitus doubles the probabilities of depression occurrence [1,16]. On the contrary, Robinson et.al compared diabetic and non-diabetic samples of patients in Great Britain and found no clear relationship between onset of diabetes and depression [17]. Comorbid depression and diabetes may significantly worsen the course of both disorders, leading to increased socioeconomic stress, reduced functioning and quality of life, and higher complication and mortality rates. Among diabetic patients, depression can be persistent and severe [18].
However, in spite of the huge impact of co-morbid depression and diabetes on the individual and its importance as a public health problem, little is known about the existence of depression in people with diabetes in Ethiopia.
The depression and diabetes are causally related and deserves attention from clinicians to ensure better management [2]. The cross sectional prevalence of type 2 diabetes and mood disorders has been increasing over the past several decades. Adverse effects of type 2 diabetes on psychiatric status and psychological well-being have been reported [4]. In Ethiopia mental Health has been one of the most disadvantaged health programs, both in terms of facilities and trained manpower. However, during the last decade; encouraging efforts have been taken to expand services throughout the country [7].
Lack of adequate understanding of the relationship between diabetes and depression is a problem that has major clinical and policy implications. In a meta-analysis of 42 studies, Anderson et al concluded that the presence of diabetes doubles the odds of having depression [8]. Patients with type 2 diabetes are 52% more likely to develop major depressive disorder (MDD) than the general population, and most of these patients are managed in primary care [12]. Comorbid depression has been shown to have a significant impact on functional disability and increase the risk of early mortality 2-3 times compared to nondepressed patients with diabetes [16].
The prevalence of depression has varied tremendously by definition, study design, source of subjects, time frame, and measurement methods in previous studies. Thus, it is difficult to accurately estimate the potential medical care needs and public health burdens of depression in the general diabetic population [19].
A number of studies, including meta-analyses, have shown the association between diabetes and depression. This is an important public health issue because depressive disorders generally have been associated with the outcomes of chronic diseases like diabetes and have contributed to the high economic burden of health care costs [20]. Current epidemiological evidence suggests that at least one third of people with diabetes suffer from clinically relevant depressive disorders [2,21,22]. For the treatment of depression in diabetes patients, it is important that depression is recognized at an early stage [23].
To date, the reasons for the higher prevalence rates of depression in diabetic patients are not yet fully understood [22,24]. So that the major aim of this study is to investigate the associated factors leading to depression among type 2 diabetic patients.
Clinical guidelines advise screening for depression in patients with diabetes [13]. But, it is difficult to accurately estimate the potential medical care needs and public health burdens of depression in the general diabetic population [19]. A timely identification of patients with sub-threshold or clinical depression and a structured approach for the management of depression in diabetes has proved to be effective in reducing the burden of depression in diabetes. In the short term, healthcare expenditure can be saved. In the long term, a better prognosis, maintenance or improvement in quality of life can be achieved in patients with diabetes, which is the ultimate goal of diabetes therapy [22]. Previous research into the prevalence of depression in diabetes has enhanced our understanding of the magnitude of the problem of depression, its ramifications and potential risk factors. However, only a few studies have been performed in specialist diabetes care settings [25].
Data on depression in the diabetes patients in Ethiopia are inadequate particularly for type 2 diabetic patients. Therefore, this study will be helpful to extend understanding of the relationship of depression to diabetes thoroughly investigating associated factors of depression in type 2 diabetic patients. Additionally, it will also help health care providers to initiate early diagnosis and management of depression based on a research finding. Besides, it provides policy makers and NGOs (Non-governmental organizations) with relevant information for future planning and interventions, and used as input for further research.
Depression and Type 2 diabetes mellitus
A cross-sectional study conducted in Chandigarh, India; discovered that from 300 type 2 diabetic patients, 68 (23%) patients fulfilled the criteria for severe depression, 54 (18%) for moderate depression and 178 (59%) patients had no clinically significant depression [2]. Prevalence estimates indicate that diabetes is associated with a twofold higher risk of comorbid depression compared to the general population, with rates among Hispanics as high as 33% [4]. Similarly, a meta-analysis by Anderson et al identified the prevalence of depression in diabetes ranging from 8 to 61% [8]. Another study showed that the rates of elevated depressive symptoms have been found to be 27% in type 2 diabetes mellitus [16].
A population study in South Australia found that the prevalence of depression in the diabetic population was 23.6% (22.1–25.1) compared with 17.1% (15.8 –18.4) in the non-diabetic population [20]. Likewise, a cross-sectional study done in Bangladesh using PHQ-9 (score ≥5) depicted that the prevalence of depressive symptoms was 34% (n=142) when using the audio questionnaire delivery method. When a cut-off value (PHQ-9 ≥10) indicative of moderate to severe depression was used, the prevalence was to be 16.5% (n=69) which was sportive of the above findings [21].
In a study from the European Depression in Diabetes (EDID) research consortium, prevalence rates for depressive affect in outpatients with diabetes ranged between 34% and 39% for Croatian out-patients,19%and 21% for Dutch out-patients and 19% and 39% for English out-patients [25]. Studies from USA and UK reported the prevalence of depression in patients with type 2 diabetic patient varying from 30 to 83% [19,26]. A small study from Iran reported 55 per cent prevalence of depression in patients with type 2 diabetic patients [27].
Diabetes may increase risk of depression because of the sense of threat and loss associated with receiving this diagnosis and the substantial lifestyle changes necessary to avoid developing debilitating complications [28]. Several reviews indicate that the prevalence of comorbid major depressive disorder (MDD) in persons with diabetes ranges from 11 to 33% and that this comorbidity is associated with high symptom burden and disability. A systematic review of 12 studies on depression compared with non-depressed persons with diabetes, the odds were three times greater than depressed persons with diabetes [29].
A quarterly publication on diabetic people care in Mexico stated that depression was twice as common in people with diabetes as in those without diabetes. Thirty one percent of people with diabetes report significant depressive symptoms and approximately 11% meet criteria for a major depressive disorder [30]. In another community based study conducted in Bangladesh, the prevalence of depression, diagnosed using the Beck Depression Inventory, was found to be 46% in patients with Type 2 diabetes in Mexico [31]. Gavard et al. reviewed 20 studies of the comorbidity of depression and diabetes and found that the prevalence of current depression in diabetic samples averaged around 15%, much higher than in the general population. Black et al. found that 31.1% of older Mexican American diabetics reported clinically significant levels of depressive symptoms [32].
Factors associated with Co-morbid Depression in Diabetic Patient
Demographic factors
There was varied study that showed different demographic associated risk factors affects development of co-morbid depression among diabetic patients. The prevalence rates of depression were significantly higher in females with type 2 diabetes mellitus compared with males with type 2 diabetes mellitus [21,30,31,33-37]. This evidence was supported by the following study finding; a doubled percentage of 41.4% than men of 17.8% [1], more than three times higher in women compared with men for the PHQ-9 (Odds ratio [OR] 3.4; 95% confidence interval [CI] 2.2-5.4) [21], women made up a significantly larger portion of the depressed subjects than those not depressed (87.5% vs. 68.9%, P=0.018) [38].
Other demographic risk factor that had significantly associated in varying degree with depression in people with diabetes includes: age at diabetes diagnosis [35,36]; younger [12,30,38], older (age > 65 years) population (40.3%) reported moderate to severe depression on PHQ-9 (P=0.008) measurement tool [13,21], age ≥54 yr (OR 1.26, 95% CI 1.02, 1.67; P<0.05) [1]; low socioeconomic status [21,30,33.34]; monthly income >5000 INR (OR 1.22, 95% CI 1.03-1.41; P<0.001) [2], with OR=0.99; P <.05 [12]; less education [9,21,30]; being unmarried [9,30]; urban residence [21]; family status; widowed and divorced individuals had higher percentages of depression (36%) in relation to married and unmarried people [1], nature of relationship with sexual partners [37], ethnicity/race; ethnic minority [39] and (with OR 2.21, 95% confidence interval (CI) 1.09, 4.46) [25]; smoking habits [14,40,41]; physical activity [14]; sedentary life [35]; and unemployment (95%) with P< 0.001 [38].
On the contrary, a study conducted by Amit et’al, Diana et’al, and Egede and Ellis found that sex/gender, age, residence, educational status, race/ethnicity, marital status, employment status, and household income had no significant association with the development of comorbid depression among diabetic patient [2,12,37,38].
Psychosocial factors
As with demographic associated risk factors, the effects of psychosocial factor on co-morbid depression in patients with type 2 diabetes mellitus supported in different studies by different scholars. These factors that had a significant association with co-morbid depression comprise; increased health care costs/financial stress [9,12,18], poor social support [9,30], experience chronic stressors or negative life events [9,12,18], poorer quality of life indices [18], pill burden [12,15].
In a survey conducted in Australia, depression was associated with poorer quality of life in all eight quality of life dimensions (physical functioning, role limitations due to impaired physical health, bodily pain, general health, vitality, social functioning, role limitations due to impaired emotional health and mental health) in patients with diabetes [22].
Medical factors
Depression was most strongly associated with physical factors; functional impairment [9], greater waist circumference (OR 1.34, 95% CI 1.04-1.64; P<0.001) [12]; biological factors; glycosylated hemoglobin (HbA1c) >8.5% [9,21], body mass index 25.0–30.0 kg ⁄m2 or obese > 30.0 kg ⁄m2 [25], >35.0 kg ⁄m2 [1], fasting blood sugar [21];diabetic complications [18]; retinopathy [42], neuropathy [9,12,25,42], nephropathy (OR 1.81, 95% CI 1.02-3.21; P=0.041) [12,42], peripheral vascular disease (OR 6.08, 95% CI 1.07-34.6; P=0.042) [12,42], diabetic foot (OR 2.32, 95% CI 1.06-5.86; P<0.001) [12], coronary vascular disease [42], ischemic heart disease [41,42], arthrosclerotic vascular disease [42]; co-morbid disease [18]; greater anxiety [18], dysthymia [18], heart disease [21]; diabetic treatment regimen; insulin or combined insulin [19], insulin or combined insulin and oral treatment [21], sexual dysfunction [42].
But on the contrary depression was not significantly associated with poor body weight, insulin treatment users, duration of diabetes, HbA1c > 8.5%, obesity (BMI > 30.0 kg⁄m2), co-morbidity (hypertension including duration), complications (retinopathy) [2,21,25,38]
Barriers to diagnose co-morbid depression
One of the major challenges in measuring depression in Bangladesh is that no depression screening tools have previously been culturally standardized for the population [21].
According to Anna M. Acee, the barriers related to screening for depression in primary care setting include time constraints, difficulties assessing depression with concurrent symptoms of diabetes, clinicians’ deficient knowledge of depression diagnosis, patient factors surrounding labeling and cultural beliefs, and current trends in managed health care. Additionally, diagnosing depression in symptomatic persons with diabetes is challenging due to the overlap in physical (e.g., weight loss and fatigue) or cognitive (e.g., trouble concentrating) symptoms [32].
Generally depression was prevalent among patients with type 2 diabetes mellitus where negative life event, poor social support, pill burden, previous depression affect, fear of complication and death, health care cost, age, sex, weight, waist circumference, educational status, marital status, income, smoking habit, physical activity, glycosylated hemoglobin (HbA1c) level, diabetes treatment regimen, diabetes complication, and co-morbid disease other than depression are the major associated risk factors. The major challenges for diagnosis of depression that was identified are lack of depression screening tool and lack of knowledge.
Hence, the purpose of this study is to ensure whether depression is common in type 2 diabetic patients or not, thoroughly identifying responsible determinant factors and challenges for its diagnosis in Ethiopian context.
1. To identify factors associated with the risk of co-morbid depression among patients with type 2 diabetes mellitus.
2. To explore factors affecting diagnosis of co-morbid depression among patients with type 2 diabetes mellitus.
Study area
Black Lion Hospital (Tikur Anbessa in Amharic), located in the nation’s capital Addis Ababa, is Ethiopia’s largest general public hospital and one of University Hospitals in the country. The hospital provides a tertiary level referral treatment and is open for 24 hours for emergency services. Black Lion hospital offers diagnosis and treatment for approximately 370,000- 400,000 patients a year [28].
There are different units and clinics that provide specialized service for clients. Among these diabetes clinics is one of which was inaugurated by Prof. Dr. Giuseppe “Pino” Grimaldi (president of the international association of lions clubs) on Saturday 12th November 1994. In diabetes unit approximately 115 type 2 diabetic patients were seen weekly.
Study design
Institution based cross-sectional study design was used to assess associated factors of depression among type 2 diabetic outpatients.
Study period
The study was conducted from April 23, 2013 to May 13, 2013.
Source population
All type 2 diabetes out-patients on follow up treatment in diabetes clinic.
Study population
Type 2 diabetes out-patients who have follow up appointment during data collection period.
Sample size determination
The actual sample size for the study was determined using the formula for single population proportion by assuming 5% marginal error (d), 95% confidence interval (alpha=0.05), and the proportion or prevalence of depression among type 2 diabetic patient was 34% (P=0.34) [21]. Based on the above information the total initial sample size was calculated by using the following formula;
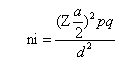
Where;
ni= required initial sample size
Za/2 =critical value for normal distribution at 95% confidence interval which equals to 1.96 (Z value at alpha=0.05).
P= Proportion of success, that is the prevalence of depressive symptoms using the PHQ-9 (score ≥5) was 34% (26) [21].
q= Proportion of type 2 diabetic population not having co-morbid depression.
d= marginal error (0.05).
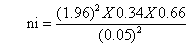
ni= 345
Since the sampling was made from finite population (N<10, 1000), it needs finite population correction. Therefore;
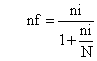
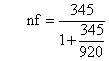
Where nf was the final sample size, ni was the initial sample size determined using the formula, and N was the size of the source population. By considering 10% non-response rate, the total sample size was 276 type 2 diabetic outpatients. Those 276 samples were selected by using systematic random sampling techniques.
Inclusion Criteria
Individuals was approached by the research team and invited to participate in the study that are;
• Diagnosed as type 2 diabetic patients for at least one year.
• Age ≥20 years old.
• Capable of independent communication and giving informed verbal consent.
• Resident physician engaged in the clinic at the time of data collection
Exclusion Criteria
• Individuals with type 1 diabetes mellitus.
• Individuals who were currently being treated for depression.
• Age <20 years old.
• Not capable of independent communication.
• Individuals who have refused to participate in the study.
Quantitative and qualitative data were collected by using structured interviewer administered questionnaire. Demographic and health related information were collected from each participant and medical records using data abstraction form. In-depth interview was conducted to collect data regarding factors affecting diagnosis of comorbid depression. Patients with established type 2 diabetes mellitus were evaluated for depression by administering the nine-item PHQ-9 (Amharic version-local language). The PHQ-9 consists of nine items on a 4-point likert-type scale. Standard cut-off scores were used with the PHQ-9 to classify minimal (1–4), mild (5–9), moderate (10-14), moderately severe (15-19), severe (20-27) symptoms of depression. Since the questionnaire was prepared in English, it was translated in to Amharic language for appropriate and easiness in interviewing the study subjects in Amharic language. The Amharic version was again translated back to English to check the consistency of meaning. Translation of questionnaire was done by language experts in both cases.
Dependent variables
• Depression
Independent variables
• Demographic factors: Age, sex, ethnicity, height, weight, waist circumference, educational status, marital status, monthly family income,
• Medical factors: diabetes treatment regimen, physical disability, co-morbid disease, body mass index, fasting blood sugar level.
• Psychosocial factors: Negative life event, poor social support, medication burden, fear of diabetic complication and death, smoking habit, high health care cost, physical activity.
Data processing and analysis
After checking collected data visually for completeness, the response was coded and entered into the computer using EPI info version 3.5.1. Statistical packages, and then 10% of the responses was randomly selected and checked for the consistency of data entry. Then printed frequencies were used for checking of outliers and to clean data. Data was cleaned accordingly and then exported to SPSS (Statistical package for Social Science) version 20.0 (IBM SPSS Corp.) for further analysis. The frequency distribution of dependent and independent variables was worked out. Multivariate logistic regression analysis model was applied to identify statistically significant associated risk factors and odds ratio was calculated to determine the strength of associations of selected variables. Further, thematic analysis was used for the qualitative data.
Data quality issues
To assure quality of the data, properly designed data collection tool was prepared and pretested and training was given to data collectors. Additionally, on each data collection day, the collected data was reviewed and checked for its completeness by principal investigator and appropriate design and sampling procedures were applied. Moreover, the exclusion criteria were considered.
Ethical Considerations
In order to follow the ethical and legal standards of the scientific investigation, the study was conducted after the approval of the proposal by Addis Ababa University institutional review board. Participation was voluntary and information was collected anonymously after obtaining verbal consent from each respondent by assuring confidentiality throughout the data collection period.
Sociodemographic characteristics
Totally 264 study participants were interviewed with a response rate of 95.6%. As shown in Table 1, of whom those interviewed participants 53.0% (n=140) were female, 69.3% (n=183) were married, 80.7% (n=213) were Orthodox Christian, and 57.2% (n=151) were Amhara. In addition, the mean ± SD age at diagnosis and current age of the subjects were 43.9 ± 10.9 and 55.9 ± 10.9 years, respectively. Besides, 86.4% (n=228) lived in Addis Ababa, and 61.7% (n=163) were with waist circumference of ≥95 cm (mean ± SD, 98.9 + 11.1). Likewise, the median monthly income of the family was 750 ETB (651-1400 ETB) and around one third of the respondents 33.7% (n=89) were attended college/university level education.
| Variable | Category | Frequency (n) | Percent (%) |
|---|---|---|---|
| Sex | Male | 124 | 47.0 |
| Female | 140 | 53.0 | |
| Residence | Addis Ababa | 228 | 86.4 |
| Outside Addis Ababa | 36 | 13.6 | |
| Current age | <=49 | 60 | 22.7 |
| 50-54 | 53 | 20.1 | |
| 55-59 | 48 | 18.2 | |
| 60-64 | 43 | 16.3 | |
| >=65 | 60 | 22.7 | |
| Age at diagnosis | <=34 | 49 | 18.6 |
| 35-39 | 45 | 17.0 | |
| 40-44 | 43 | 16.3 | |
| 45-49 | 49 | 18.6 | |
| 50-54 | 27 | 10.2 | |
| >=55 | 51 | 19.3 | |
| Marital status | Single | 9 | 3.4 |
| Married | 183 | 69.3 | |
| Divorced | 24 | 9.1 | |
| Widowed | 48 | 18.2 | |
| Religion | Orthodox | 213 | 80.7 |
| Muslim | 24 | 9.1 | |
| Protestant | 20 | 7.6 | |
| Catholic | 2 | 0.8 | |
| Others | 5 | 1.9 | |
| Ethnicity | Amhara | 151 | 57.2 |
| Oromo | 40 | 15.2 | |
| Tigre | 25 | 9.5 | |
| Gurage | 29 | 11.0 | |
| Others | 19 | 7.2 | |
| Educational status | Can’t read & write | 35 | 13.3 |
| Read and write only | 16 | 6.1 | |
| Primary school(1-8) | 65 | 24.6 | |
| Secondary( 9-12) | 59 | 22.3 | |
| College/University | 89 | 33.7 | |
| Occupation | Farmer | 6 | 2.3 |
| Civil servant | 47 | 17.8 | |
| Merchant | 10 | 3.8 | |
| House wife | 47 | 17.8 | |
| Private worker | 38 | 14.4 | |
| Pensioned | 58 | 22.0 | |
| No employment | 48 | 18.2 | |
| Others | 10 | 3.8 | |
| Monthly family income (ETB) | <=650 | 125 | 47.3 |
| 651-1400 | 61 | 23.1 | |
| >=1401 | 78 | 29.5 | |
| Waist circumference (cm) | <95 | 101 | 38.3 |
| >=95 | 163 | 61.7 |
Table 1: Sociodemographic characteristics of type 2 diabetic outpatient in Black Lion General Specialized Hospital, Addis Ababa, Ethiopia, June, 2013. (n=264).
Clinical characteristics
As illustrated in Table 2, 43.2% (n=145) were on oral hypoglycemic treatment, 78.3% (n=141) were with cardiovascular diseases (hypertension and heart failure), and 69.7% (n=140) were with diabetic retinopathy. Above and beyond, more than half (58.7%, n=155) of study participants reported 1 to 2 co-morbid disease (mean ± SD, 1.1 ± 0.9) i.e. evidenced by review of patient’s medical record. Similarly, half of the respondents 50% (n=132) were with BMI ≤24.9 kg/m2 (mean ± SD, 25.4 ± 3.7) and reported physical disability and more than one third were living with diabetes mellitus and taking physician prescribed medication for ≥8 years (mean ± SD, 12 ± 7.9). Regarding the laboratory reported fasting blood glucose level, 12.9% (n=34) were with ≤100 mg/ dl, 19.7% (n=52) were with 101-126 mg/dl, and 67.4% (n=178) were with ≥127 mg/dl (Figure 1).
| Variable | Category | Frequency | Percent (%) |
|---|---|---|---|
| Diabetes treatment regimen | Single insulin | 108 | 40.9 |
| Combined insulin | 12 | 4.5 | |
| Insulin plus oral hypoglycemic | 30 | 11.4 | |
| Oral hypoglycemic | 114 | 43.2 | |
| Duration of diabetes (years) | <= 8 | 101 | 38.3 |
| 9 – 16 | 96 | 36.4 | |
| 17+ | 67 | 25.4 | |
| Duration of diabetes treatment (years) | <= 8 | 105 | 39.8 |
| 9 – 16 | 93 | 35.2 | |
| 17+ | 66 | 25.0 | |
| Co-morbid disease a | Cardiovascular disease | 141 | 78.3% |
| Respiratory disease | 17 | 9.4% | |
| Renal disease | 13 | 7.2% | |
| Neurologic disease | 4 | 2.2% | |
| Other co-morbid disease | 80 | 44.4% | |
| Complication of diabetes b | Diabetic retinopathy | 140 | 69.7% |
| Diabetic nephropathy | 69 | 34.3% | |
| Diabetic neuropathy | 83 | 41.3% | |
| Sexual dysfunction | 69 | 34.3% | |
| Body mass index | <=24.9 | 132 | 50.0 |
| 25.0-29.9 | 98 | 37.1 | |
| >=30 | 34 | 12.9 | |
| Fasting blood glucose | <=100 | 34 | 12.9 |
| 101-126 | 52 | 19.7 | |
| >=127 | 178 | 67.4 | |
| Number of co-morbidity | 0 | 83 | 31.4 |
| 1-2 | 155 | 58.7 | |
| >=3 | 26 | 9.8 | |
| Number of prescribed medication administration per day | <= 4 | 77 | 29.2 |
| 5 – 6 | 113 | 42.8 | |
| 7+ | 74 | 28.0 | |
| Number of diabetic complication | 0 | 65 | 24.6 |
| 1-2 | 148 | 56.1 | |
| >=3 | 51 | 19.3 | |
| Physical disability | Yes | 132 | 50.0 |
| No | 132 | 50.0 | |
| Medication burden | < 3 | 77 | 29.2 |
| >4 | 187 | 70.8 |
a= number of respondent is 180, b= number of respondent is 201
Table 2: Clinical characteristics of type 2 diabetic outpatient in Black Lion General Specialized Hospital, Addis Ababa, Ethiopia, June, 2013. (n=264).
Psychosocial factors
As depicted in Figure 2, three fourth of the study participants (75.6%, n=192) were reported that health care cost for diabetes treatment (type 2) was high and one fifth (22%, n=55) of the study respondents do physical activity as recommended by the physician.
Reliability and item analysis
Cronbach’s α for the PHQ-9 scale was 0.72 indicating acceptable consistency of this psychometric scale for the study population. The correlations between nine items of the PHQ-9 and total PHQ-9 scores ranged from 0.22 to 0.69, and all correlations were significant at the 0.01 level.
Factors associated with depression
Tables 3-5 illustrated the results of logistic regression analysis examining the associations between demographic, clinical, and psychosocial factors and depression symptoms.
The prevalence of depressive symptoms were more than three times higher in study participants of monthly family income ≤650 ETB compared with monthly family income ≥1401 ETB (COR 3.4; 95% CI 1.28-8.87; p-value 0.01). This was found statistically significant. Other demographic variables that found risk for depression, but not statistically significant were female sex, residence outside Addis Ababa, religion (muslim and protestant), ethnicity, educational status, monthly family income (651-1400 ETB), current age (≤49 and 55-59 years), and age at diagnosis (Table 3). Among clinical and psychosocial variables, diabetic retinopathy (OR 2.6, 95% CI 1.09-6.31, p-value 0.03), number of diabetic complication from 1 to 2 (OR 2.5, 95% CI 1.04-6.19, p-value 0.04), and poor social support (OR 15.5, 95% CI 2.07-116.95, p-value 0.01), were all found to be statistically significant with a greater risk of depression (Tables 4 and 5).
| Variable and Categories |
Depression(n) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| Yes | No | p-Value | OR(95% CI) | p-Value | OR(95% CI) | |
| Sex | ||||||
| Male* | 109 | 15 | ||||
| Female | 130 | 10 | 0.17 | 1.8(0.77-4.14) | 0.39 | 1.6(0.54-4.95) |
| Residence | ||||||
| Addis Ababa* | 204 | 24 | ||||
| Outside Addis Ababa | 35 | 1 | 0.17 | 4.1(0.54-31.42) | 0.17 | 4.6(0.51-42.76) |
| Religion | ||||||
| Orthodox* | 192 | 21 | ||||
| Muslim | 22 | 2 | 0.81 | 1.2(0.26-5.48) | 0.47 | 2.0(0.30-12.99) |
| Protestant | 19 | 1 | 0.49 | 2.1(0.26-16.32) | 0.34 | 3.2(0.29-35.38) |
| Catholic** | 2 | 0 | - | - | - | - |
| Others | 4 | 1 | 0.47 | 0.4(0.05-4.10) | 0.20 | 0.1(0.01-2.88) |
| Marital status | ||||||
| Single | 8 | 1 | 0.61 | 0.5(0.05-5.79) | 0.59 | 0.7(0.03-7.43) |
| Married | 166 | 17 | 0.51 | 0.6(0.18-2.32) | 0.93 | 0.9(0.19-4.62) |
| Divorced | 20 | 4 | 0.17 | 0.3(0.07-1.63) | 0.13 | 0.2(0.03-1.54) |
| Widowed* | 45 | 3 | ||||
| Ethnicity | ||||||
| Amhara* | 135 | 16 | ||||
| Oromo | 37 | 3 | 0.56 | 1.5(0.40-5.29) | 0.89 | 0.9(0.19-4.06) |
| Tigre | 23 | 2 | 0.69 | 1.4(0.29-6.33) | 0.32 | 2.6(0.39-17.19) |
| Gurage | 26 | 3 | 0.97 | 1.0(0.28-3.78) | 0.60 | 0.6(0.13-3.30) |
| Others | 18 | 1 | 0.47 | 2.1(0.27-17.06) | 0.60 | 2.0(0.15-26.11) |
| Educational status | ||||||
| Can’t read &write | 32 | 3 | 0.37 | 1.8(0.49-6.84) | 0.89 | 0.9(0.16-4.96) |
| Read and write only** | 16 | 0 | - | - | - | - |
| Grade 1-8 | 62 | 3 | 0.06 | 3.5(0.96-12.96) | 0.24 | 2.6(0.53-13.13) |
| Grade 9-12 | 53 | 6 | 0.43 | 1.5(0.54-4.23) | 0.89 | 1.1(0.32-3.77) |
| College/University* | 76 | 13 | ||||
| Monthly family income (ETB)*** | ||||||
| <650 | 119 | 7 | 0.01 | 3.4(1.28-8.87) | 0.09 | 3.4(0.83-13.76) |
| 651-1400 | 56 | 5 | 0.15 | 2.2(0.75-6.67) | 0.14 | 2.7(0.72-10.29) |
| >1401* | 65 | 13 | ||||
| Waist circumference | ||||||
| <95cm* | 92 | 9 | ||||
| >95cm | 147 | 16 | 0.81 | 0.9(0.38-2.12) | 0.66 | 0.8(0.28-2.21) |
| Current age | ||||||
| <=49 | 56 | 4 | 0.35 | 1.8(0.51-6.68) | 0.54 | 1.9(0.24-15.73) |
| 50-54 | 47 | 6 | 0.95 | 1.0(0.32-3.29) | 0.97 | 0.9(0.18-5.15) |
| 55-59 | 47 | 1 | 0.09 | 6.2(0.74-52.32) | 0.06 | 9.8(0.9-106.57) |
| 60-64 | 38 | 7 | 0.50 | 0.7(0.22-2.10) | 0.57 | 0.7(0.16-2.7) |
| >65* | 53 | 7 | ||||
| Age at diagnosis | ||||||
| <=34 | 44 | 5 | 0.80 | 1.2(0.33-4.13) | 0.70 | 0.7(0.09-4.85) |
| 35-39 | 43 | 2 | 0.21 | 2.8(0.55-14.99 | 0.42 | 2.5(0.26-24.53) |
| 40-44 | 39 | 4 | 0.70 | 1.3(0.34-4.94) | 0.76 | 0.7(0.11-4.9) |
| 45-49 | 44 | 5 | 0.80 | 1.2(0.33-4.13) | 0.82 | 0.8(0.15-4.43) |
| 50-54 | 24 | 3 | 0.93 | 1.1(0.24-4.65) | 0.91 | 1.9(0.16-4.98) |
| >55* | 45 | 6 | ||||
*=reference category; **=collinear (numerical problem; ***=1 US$, 18 Ethiopian birr, p-value=significant, <0.05; OR= Odds Ratio
Table 3: Logistic regression examining the associations between demographic factors and depression symptoms among type 2 diabetic outpatients in Black Lion
General Specialized Hospital, Addis Ababa, Ethiopia, June, 2013.
| Variable and Categories | Depression(n) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| Yes | No | p-Value | OR(95% CI) | p-Value | OR(95% CI) | |
| Diabetes treatment regimen | ||||||
| Single insulin* | 97 | 11 | ||||
| Combined insulin | 10 | 2 | 0.49 | 0.6(0.11-2.93) | 0.50 | 0.5(0.07-3.68) |
| Insulin plus oral hypoglycemic | 29 | 1 | 0.26 | 3.3(0.41-26.55) | 0.38 | 2.7(0.29-26.08) |
| Oral hypoglycemic | 103 | 11 | 0.89 | 1.1(0.44-2.56) | 0.57 | 1.4(0.42-4.88) |
| Co-morbidity | ||||||
| Cardiovascular disease(no*) | 129 | 12 | 0.57 | 1.3(0.56-2.89) | 0.32 | 0.3(0.03-3.33) |
| Respiratory disease(no*)** | 17 | 0 | - | - | - | - |
| Renal disease(no*) | 12 | 1 | 0.82 | 1.3(0.16-10.18) | 0.64 | 0.5(0.04-6.76) |
| Neurologic disease(no*)** | 4 | 0 | - | - | - | - |
| Other co-morbid disease(no*) | 77 | 3 | 0.05 | 3.5(1.01-12.00) | 0.52 | 1.7(0.32-9.77) |
| Diabetic complication | ||||||
| Diabetic retinopathy(no*) | 132 | 8 | 0.03 | 2.6(1.09-6.31) | 0.33 | 1.9(0.52-6.87) |
| Diabetic nephropathy(no*) | 64 | 5 | 0.46 | 1.5(0.53-4.06) | 0.58 | 0.6(0.14-3.01) |
| Diabetic neuropathy(no*) | 78 | 5 | 0.20 | 1.9(0.70-5.36) | 0.79 | 1.2(0.26-5.80) |
| Sexual dysfunction(no*) | 63 | 6 | 0.79 | 1.1(0.43-2.97) | 0.99 | 1.0(0.26-3.95) |
| Duration of diabetes | ||||||
| < 8* | 90 | 11 | ||||
| 9 – 16 | 90 | 6 | 0.25 | 1.8(0.62-5.17) | ||
| 17+ | 59 | 8 | 0.83 | 0.9(0.34-2.37) | ||
| Duration of diabetes treatment | ||||||
| < 8* | 94 | 11 | ||||
| 9 – 16 | 87 | 6 | 0.32 | 1.7(0.60-4.78) | ||
| 17+ | 58 | 8 | 0.74 | 0.8(0.32-2.23) | ||
| Fasting blood sugar (mg/dl) | ||||||
| < 100mg/dl* | 29 | 5 | ||||
| 101-126 | 44 | 8 | 0.93 | 0.9(0.28-3.18) | 0.52 | 0.6(0.15-2.66) |
| >=127 | 166 | 12 | 0.13 | 2.4(0.78-7.27) | 0.37 | 1.8(0.51-6.14) |
| Body mass index | ||||||
| < 24.9* | 118 | 14 | ||||
| 25.0-29.9 | 89 | 9 | 0.72 | 1.2(0.48-2.83) | 0.93 | 1.1(0.38-2.85) |
| >=30 | 32 | 2 | 0.41 | 1.9(0.41-8.79) | 0.56 | 1.7(0.30-9.01) |
| Number of co-morbidity | ||||||
| 0* | 71 | 12 | ||||
| 1-2 | 143 | 12 | 0.11 | 2.0(0.86-4.71) | 0.14 | 6.7(0.53-86.29) |
| >3 | 25 | 1 | 0.18 | 4.2(0.52-34.17) | ||
| Number of prescribed medication administration per day | ||||||
| < 4* | 89 | 8 | ||||
| 5 – 6 | 101 | 12 | 0.96 | 0.9(0.38-2.51) | 0.39 | 0.52(0.12-2.31) |
| 7+ | 69 | 5 | 0.43 | 1.6(0.49-5.13) | 0.71 | 0.70(0.11-4.51) |
| Number of diabetic complication | ||||||
| 0* | 54 | 11 | ||||
| 1-2 | 137 | 11 | 0.04 | 2.5(1.04-6.19) | 0.26 | 2.5(0.52-11.63) |
| >3 | 48 | 3 | 0.08 | 3.3(0.86-12.38) | 0.79 | 1.5(0.07-33.85) |
| Physical disability | ||||||
| Yes | 124 | 8 | 0.06 | 2.3(0.95-5.51) | 0.40 | 1.6(0.53-4.93) |
| No* | 115 | 17 | ||||
*=reference category; **=collinear (numerical problem; p-value=significance level, <0.05; OR= Odds Ratio
Table 4: Logistic regression examining the associations between clinical factors and depression symptoms among type 2 diabetic outpatients in Black Lion General Specialized Hospital, Addis Ababa, Ethiopia, June, 2013.
| Variable and Categories | Depression(n) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| Yes | No | p-Value | OR(95% CI) | p-Value | OR(95% CI) | |
| Negative life event | ||||||
| Yes | 81 | 7 | 0.55 | 1.32(0.53-3.28) | 0.85 | 0.9(0.35-2.39) |
| No* | 158 | 18 | ||||
| Poor social support | ||||||
| Yes | 94 | 1 | 0.01 | 15.5(2.07-117) | 0.01 | 14.7(1.94-111.89) |
| No* | 145 | 24 | ||||
| Fear of diabetic complication | ||||||
| Yes | 164 | 14 | 0.73 | 0.7(0.08-5.57) | 0.83 | 0.8(0.09-6.69) |
| No | 58 | 10 | 0.32 | 0.3(0.04-2.86) | 0.49 | 0.5(0.05-4.19) |
| I don’t know* | 17 | 1 | ||||
| High health care cost | ||||||
| Yes | 174 | 18 | 0.59 | 0.6(0.07-4.53) | 0.59 | 0.6(0.07-4.69) |
| No | 48 | 6 | 0.50 | 0.5(0.05-4.19) | 0.57 | 0.5(0.06-4.86) |
| I don’t know* | 17 | 1 | ||||
| Physical activity | ||||||
| Yes | 49 | 7 | 0.38 | 0.7(0.26-1.68) | 0.75 | 0.8(0.31-2.33) |
| No | 190 | 18 | ||||
| Medication burden | ||||||
| < 3* | 89 | 8 | ||||
| > 4 | 170 | 17 | 0.74 | 1.2(0.48-2.81) | 0.73 | 1.2(0.47-3.05) |
*= reference category, p-value= significance level, p<0.05, OR= Odds Ratio
Table 5: Logistic regression examining the associations between psychosocial factors and depression symptoms among type 2 diabetic outpatients in Black Lion General Specialized Hospital, Addis Ababa, Ethiopia, June, 2013.
However, only poor social support was a statistically significantly associated risk factor for depression after controlling of other confounding factors using multivariate logistic regression analysis model (Table 5).
Barriers of diagnosis of co-morbid depression
To explore the barriers of diagnosis of co-morbid depression among type 2 diabetic outpatients, in-depth interviews were done among 10 resident physicians in diabetic clinic. The mean year of experience of respondents was 7 years. Seven of 10 respondents didn’t screen depression among type 2 diabetic outpatients. But three respondents consider screening of depression for type 2 diabetic outpatients. From these three respondents who was screening depression one respondent replied, “I was considering screening of depression for type 2 diabetic outpatients during their follow-up treatment. To evaluate the presence or absence of depression I used patient verbalization/complaints of depressive symptoms, if I suspect the presence of depressive symptoms I probe some question, but I didn’t do any further examination”
Among the seven respondents who didn’t screen depression among type 2 diabetic outpatients one respondent said, “I didn’t know that depression should be screened for type 2 diabetic patients”. The other mentioned reasons by the rest of respondents were;
• I am not a psychiatrist so I am not responsible to diagnose mental illnesses, only I focus on medical problems
• There was no standardized assessment tool
• There was a well-organized referral psychiatric clinic in the hospital that do further evaluation
• Patients are not complaining the symptoms of depression
• Shortage of time and patient overload
It is difficult to accurately estimate the potential medical care needs and public health burdens of depression in the general diabetic population [24]. However, in spite of the huge impact of co-morbid depression and diabetes on the individual and its importance as a public health problem, little is known about the existence of depression in people with diabetes in Ethiopia. This study has tried to address this issue.
The aim of this study was to identify sociodemographic, clinical, psychosocial factors associated with depression and barriers to diagnose depression in type 2 diabetic outpatients. The prevalence of depression was significantly associated with low socio economic status [21,30,34,35]. A study conducted by Amit et’al, Diana et’al, and Egede and Ellis found that sex/gender, age, residence, educational status, race/ ethnicity, marital status, and employment status had no significant association with the development of co-morbid depression among diabetic patient [4,14,38]. In this study a univariate logistic regression analysis result showed that monthly family income ≤650 ETB (OR 3.4, 95% CI 1.28-8.87, p-value 0.01) were a statistically significant associated risk factor for depression in type 2 diabetic patients. But, female sex (OR 1.8, 95% CI 0.77-4.14, p-value 0.17), Addis Ababa residence (OR 4.1, 95% CI 0.54-31.42, p-value 0.17), protestant religion (OR 2.1, 95% CI 0.26-16.32, p value 0.49), others ethnicity (OR 2.1, 95% CI 0.27-17.06, p-value 0.47), primary school (1-8) educational level (OR 3.5, 95% CI 0.96-12.96, p-value 0.06), monthly family income 651-1400 ETB (OR 2.2, 95% CI 0.75-6.67, p-value 0.15), current age 55-59 year (OR 6.2, 95% CI 0.74-52.32, p-value 0.09), and age at diagnosis 35-39 year (OR 2.8, 95% CI 0.55-14.99, p-value 0.21) were risk factors associated with high depression in type 2 diabetic outpatients, even though they are not statistically significant. This means the observed association occurred by chance.
However, handful of earlier published articles reported the prevalence rates of depression were significantly higher in females with type 2 diabetes mellitus compared with males with type 2 diabetes mellitus [21,30,31,34,35,37-42]. Other demographic risk factor that had significantly associated in varying degree with depression in people with diabetes includes: age at diabetes diagnosis [37]; younger [14,30,38], older (age > 65 years) population (40.3%) [16,21], age ≥54 yr (OR 1.26, 95% CI 1.02, 1.67; P<0.05) [1]; less education [10,21,30]; being unmarried [10,30]; urban residence [21]; family status; widowed and divorced individuals had higher percentages of depression (36%) [1], ethnicity/race; physical activity [17]; and unemployment (95%) with P< 0.001 [38].
Regarding the clinical characteristics of participants, earlier studies conducted in different setting revealed depression was most strongly associated with physical factors; functional impairment [10], greater waist circumference (OR 1.34, 95% CI 1.04-1.64; P<0.001) [14]; biological factors; body mass index (25.0–30.0 kg ⁄m2) or obese > 30.0 kg ⁄m2 [25], >35.0 kg ⁄m2 [1], fasting blood sugar [21];diabetic complications [18]; retinopathy [33], neuropathy [33], with (OR 1.94, 95% CI 1.03-3.66; P=0.002) [10, 14], with (OR 3.3, 95% CI 1.8, 5.9) [25], and with (P < 0.001) 52% [25] , nephropathy (OR 1.81, 95% CI 1.02-3.21; P=0.041) [14,33], co-morbid disease [18]; heart disease [21]; diabetic treatment regimen; insulin or combined insulin [24], insulin or combined insulin and oral treatment [21], and sexual dysfunction [33].
Similarly, the result of this study demonstrated a significantly higher prevalence of depression in type 2 diabetic outpatients with diabetic retinopathy (OR 2.6, 95% CI 1.09-6.31, p-value 0.03) and number of diabetic complication from 1 to 2 (OR 2.5, 95% CI 1.04- 6.19, p-value 0.04). Which means the likelihood of depression was increased in those populations with the presence of the above variables. But on the contrary depression was not significantly associated with insulin treatment users, duration of diabetes, obesity (BMI > 30.0 kg ⁄m2), co-morbidity (hypertension including duration), complications (retinopathy) [4,21,25,38].
In this study insulin plus oral hypoglycemic treatment regimen (OR 3.3, 95% CI 0.41-26.55, p-value 0.26), fasting blood sugar ≥127 mg/ dl (OR 2.4, 95% CI 0.78-7.27, p-value 0.13), number of co-morbidity ≥3 (OR 4.2, 95% CI 0.52-34.17, p-value 0.18), number of diabetic complication ≥3 (OR 3.3, 95% CI 0.86-12.38, p-value 0.08), and physical disability (OR 2.3, 95% CI 0.95-5.51, p-value 0.06) were risk factors associated with presence of high depressive symptoms in type 2 diabetic outpatients, but not statistically significant. This means the observed association also occurred by chance. Which means the likelihood of depression was increased in those populations with the presence of the factors. However, the relation was not casual. These factors are not independent risk factors after controlling other confounding factors using multivariate logistic regression model. The rest of other variables (OR < 1, p value > 0.05) are protective to depression in type 2 diabetic patients, i.e. means the presence of these factors decrease the likelihood of depressive symptom occurrence. In a survey conducted in Australia, depression was associated with impaired physical health, bodily pain, vitality, social functioning, role limitations due to impaired emotional health and mental health) in patients with diabetes [23].
In this study poor social support (OR 15.5, 95% CI 2.07-116.95, p-value 0.01) were statistically significant associated risk factors for depression. In line with this study the factors that had a significant association with co-morbid depression comprise; increased health care costs/financial stress [10,14,18], poor social support [10,30], experience chronic stressors or negative life events [10,14,18], pill burden [14,19].
In general, the difference in association of respondents’ characteristics and co-morbid depression was might be due to variation in study design, demographic characteristics of respondents, selection method of respondents, level of country development, time frame, setting, and life style variation. From thematic analysis of in-depth interview data, the major barriers to diagnose depression among type 2 diabetic outpatients that were explored are absence of standardized screening tool and physicians give special attention only for medical problems. This leads to lack of psychological support in diabetes clinic. This finding was in line with the study conducted in Bangladesh, showed that there were no depression screening tools that have previously been culturally standardized for the population [21]. Similarly in the study conducted in UK, the authors concluded that expert psychological support was not available to the majority of diabetes centres [29].
The findings of this study have major implications for clinical practice in Black Lion General Specialized Hospital and other health care setting, where physicians’ recognition of mental disorder rates is low and improving recognition rates is a challenge because of the high patient loads and poor undergraduate training in these skills. Providing the patients with the results of blood sugar, cholesterol, blood pressure and medications plan through outpatient service is not enough itself to improve service delivery and bring about change [21].
The strengths of this study include a high response rate and the inclusive nature of this research as individuals could participate regardless of literacy level. Including patients from different ethnic backgrounds in Addis Ababa and outside Addis Ababa was a further strength. Additionally, rather than having to rely on self-report, health related information was collected from patients’ medical records. Even though the association was temporary, depression and type 2 diabetes mellitus were causally related and deserves attention from clinicians to ensure better management. Also, a reasonable sample size and ascertaining depression with culturally standardized questionnaires are strengths of this study. Since it was the first study in type, it will provide basic information for those who are interested.
However, an important limitation of this study was that a psychiatric diagnostic interview which is considered as the gold standard for the diagnosis of depression was not used. Above and beyond, there was absence of similar study done in Ethiopia health care setting to compare the finding. Moreover, due to cross-sectional nature of the study, causal relationships between depression and type 2 diabetes mellitus could not be assumed
In conclusion, this study demonstrated that depression is a common co-morbid health problem in type 2 diabetic out-patients in Ethiopia. Within this sample of out-patients with type 2 diabetes mellitus, the study found that low socioeconomic status, diabetic retinopathy, number of complication from 1 to 2 and poor social support were risk factors for depression symptoms. However, poor social support was persisted as determinant factor for co-morbid depression after controlling for other confounding factors. The major challenges for diagnosis of depression that was identified are lack of depression screening tool, presence of well-organized separated referral psychiatric clinic, patients are not complaining the symptoms of depression, and physicians did not consider as a responsibility.
This study provides rich data on the associated factors of depression in type 2 diabetic outpatients in Ethiopia. In a setting where recognition, screening and treatment levels remain low, health care providers need to focus their efforts on diagnosing, referring and effectively treating co-morbid depression in order to deliver rights-based and clientcentered services for people in real needs. Additionally, the hospital is better to prepare/adopt a standardized depression screening tool and inform physicians that they are responsible to screen and treat promptly. Teaching institutions better to incorporate the issue in curriculum to increase their knowledge about the relationship. Besides, students (graduate or undergraduate) better to do longitudinal study to determine the statistically significant associated factors for depression among patients with type 2 diabetes mellitus.
We would like to address our sincerest and heart –felt gratitude to our advisors Yosief Tsige (MSN) and Dr. Abdurazak Ahmed (MD) for their concrete and expert advice, suggestions and assistance in all aspects of this research work.
Our in-depth gratitude also goes to Addis Ababa University, College of Health Science, School of Allied Health science, Department of Nursing and Midwifery Institutional Review Board, for giving this chance and financial support too.
We would like to thank Dr. Yewondweson Tadesse, department head of Internal Medicine; Dr. Tedilla, Physicians coordinator in diabetes clinic; Sr. Abeba Mulugeta, Nurses coordinator in diabetic clinic; in Black Lion General Specialized Hospital for their collaboration to permit and facilitate data collection.
Supervisors, data collectors and respondents were highly acknowledged for investing their precious time in supervising, collecting data and providing the necessary information.
We would like to offer our great respect and appreciation to all our friends and senior instructors who gave us precious time for advice and comments during data entry and analysis.