Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Short Communication - (2018) Volume 7, Issue 2
The Perkin reaction is an organic reaction which is used for synthesis of α, β-unsaturated aromatic acid by the condensation of an aromatic aldehyde and an acid anhydride, in the presence of an alkali salt of the acid. The alkali salt acts as a base catalyst and other bases can be used instead.
Keywords: Perkin reaction; Condensation reaction; Unsaturated carboxylic acid; Acidic anhydride; Aldehyde; Aromatic aldehyde
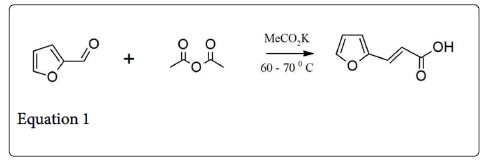
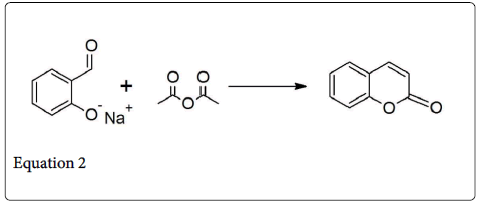
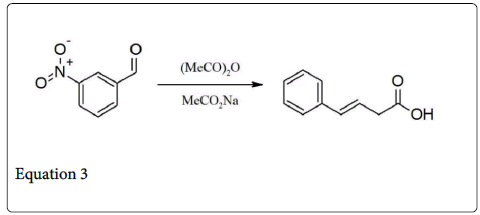
It is type of condensation reaction, which involve the condensation of acidic anhydride and aldehyde in the presence of weak base (i.e., Sodium and potassium salt of the acid or trimethylamine) to give unsaturated carboxylic acid (Equation 1) [1]. In 1968 Perkin described the very first example of such type condensation reaction, involve the synthesis of coumarin by condensing the sodium or potassium salt of salicylaldehyde with acetic anhydride (Equation 2) [2]. Generally, such type of reaction is only applicable to aromatic aldehyde and useful for the preparation of substituted cinnamic acid (Equation 3) [3].
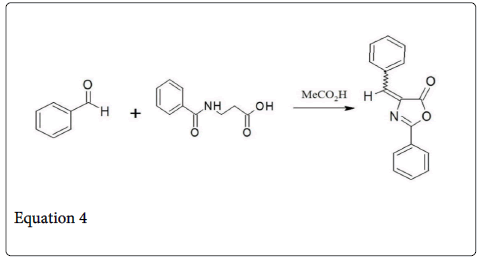
In 1883 a very important variation is done by plӧchl, which involve the heating of benzaldehyde and hippuric acid in presence of acetic anhydride [4]. Erlenmeyer determine the Azalactone structure of the product (Equation 4) and extended the scope of Perkin reaction to other aldehydes (Erlenmeyer Azalactone synthesis) [5-10]. Azalactone or oxazolone acts as important intermediate for the synthesis of α- amino acid and α- keto acid (Scheme 1).
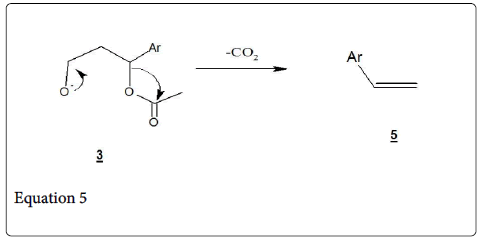
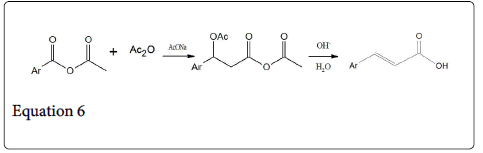
The most accepted mechanism of Perkin reaction is shown in Scheme 2a [1] and Scheme 2b [11]. Formation of anhydride enolates (1) and aldol type condensation provides the alkoxide anhydride (2) but intermolecular acylation generates an Acetoxy carboxylate (3), Which form a mixed anhydride which on elimination of acetic acid and subsequent hydrolysis gives the unsaturated acid (5) (Cinnamic acid). The compound 3 undergoes a minor potential side reaction decarboxylation to form an alkene (equation 5, 6).
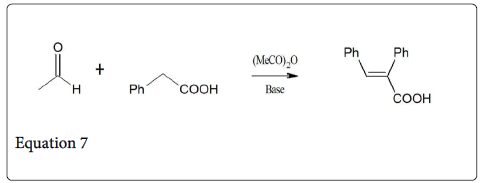
The reaction between aromatic aldehyde and phenyl acetic acid give the α-phenylcinnamic acid in cis-form (equation 7) [12]. When this product i.e., α-phenylcinnamic acid is heated in dilute solution of acetic anhydride- trimethylamine gives an equilibrium mixture of 81% of the E-cinnamic acid and 19% of the Z isomer (equation 8).
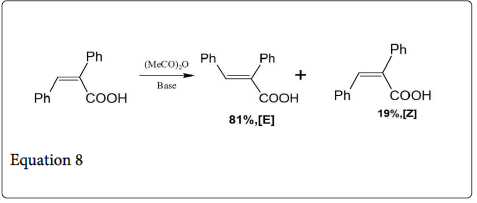
According to Ahramjian and Zimmerman, the condensation between the aromatic aldehyde and phenylacetic acid is not reversible [12]. This lack of reversibility is explained the rapid acetylation of the β-alkoxide substituent in the intermediate (2; Scheme 2). The elimination of each diasteriomeric-2, 3-diphenylpropionic acid (6, 7) under Perkin condensation ((MeCO)2O, Et3N, reflux,35 min) give a product having 99+2% of α-phenyl-trans-cinnamic acid (8; Scheme 3). Under the same mild condition, the Perkin condensation between benzaldehyde and phenyl acetic acid gives the product having 96% of (8).
Recently, many results have been published showing that the initial condensation at least in presence of trimethylamine, may not be only aldol type but also indicate a pathway involving the formation and subsequent cycloaddition of ketene to form a β-lactone intermediate that breaks to give the cinnamic acid (11, Scheme 4) [13].
Spectroscopic data (IR, NMR) is provided for the formation of β- lactone intermediate (as suggested in 1936 by Hurd and Williams) in the reaction of P- nitrobezaldehyde with ketene to give Perkin product, Cinnamic acid, anhydride and trimethyl amine was discovered and β- lactone was noticed by IR-spectroscopy under these conditions. Similar result was investigated earlier related to Perkin reaction of Quinone [14]. When 2, 6-dimethoxy-p-bezoquinone treated with propionic anhydride in presence of sodium propionate gives a mixture of condensed product (12) and (13).
Although, when the same reaction is carried out in milder condition, the product (14) is isolated and it can be converted to 12 and 13 in Perkin condition. It is observed that (14) may be in equilibrium with dimethoxybenzoquinone and methyl ketene. The isolation of product (14) does not prove that the product (14) is the reaction intermediate of product (12) and (13). So, we can conclude that the mechanism of the Perkin reaction is not as simple as indicated in Scheme 2a and Scheme 2b. It may have more than the mentioned steps; it depends upon the nature of reacting species and base (Scheme 5).
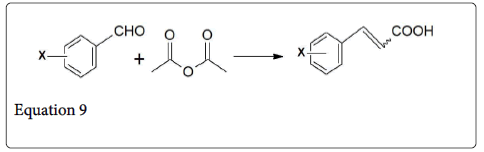
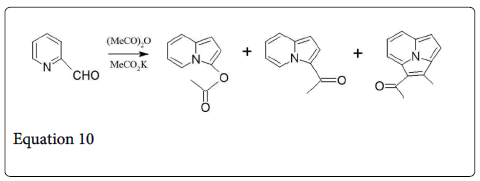
The Perkin reaction is a primary method for the synthesis of cinnamic acid derivatives (equation 9) and many examples are listed in Table 1 [1,15,16]. The aromatic aldehydes pthalaldehyde [17], isophthalaldehyde [18], and terepthaldehyde [19] also undergo such type of condensation reaction and gives benzendediacrylic acid in 28-80% yields. Heteroaromatic aldehydes such as furfural [20] (equation 1) and 2-thiophenecarbaldehyde [21] also take part in this reaction. On the other hand, 2-pyridinecarbaldehyde do not give Perkin product 3-(-pyridyl) acrylic acid [22] but it gives the mixture of indolizine derivatives (equation 10) [1].
| S. N. | Substituent | Yield Cinnamic acid (%) |
|---|---|---|
| 1. | H | 70-75 |
| 2. | 4-Me | 33 |
| 3. | 2-Cl | 71 |
| 4. | 4-Cl | 52 |
| 5. | 2-MeO | 55 |
| 6. | 4-MeO | 30 |
| 7. | 2-NO2 | 75 |
| 8. | 4-NO2 | 82 |
| 9. | 2,6-Cl2 | 82 |
Table 1: Preparation of cinnamic acid from substituted Benzaldehydes.
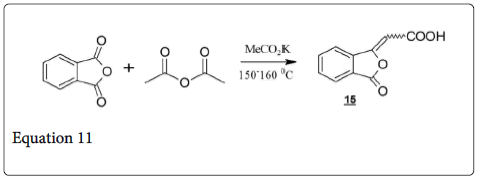
Under the Perkin reaction condition when pthalic anhydride treated with acetic anhydride and potassium acetate, gives phthalylacetic acid (15; equation 11).
According to Crawford and Little [23], simple aliphatic and aromatic ketones and aliphatic aldehydes are not generally acceptable components in the Perkin transformation. Because of increased hindrance of side chain higher aldehydes and cis-citral the reactivity of carbonyl group decreases and finally do not affords Perkin product. The aliphatic aldehydes are less reactive than the aromatic aldehydes because in elimination steps is facilitated by conjugation of newly forming double bond and the aromatic π-system in transition state.
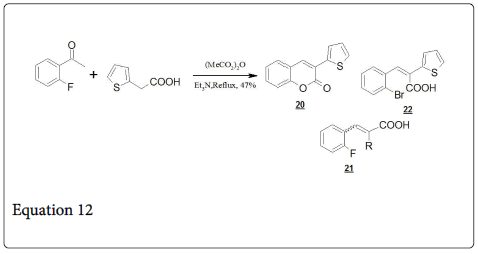
The condensation of O-hydroxybenzaldehydes is an important method for the synthesis of Coumarins (Equation 2). But the heating a mixture of O-fluorobenzaldehyde, 2-thiopheneacetic acid, acetic anhydride and trimethylamine gives directly the Coumarins [24] (20, Equation 12) instead of expected cinnamic acid 21.