Drug Designing: Open Access
Open Access
ISSN: 2169-0138
ISSN: 2169-0138
Market Analysis - (2020)
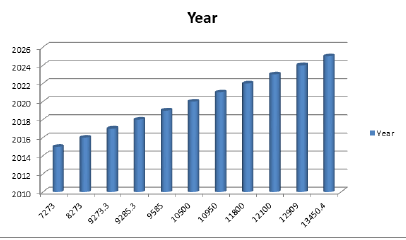
The worldwide clinical trials advertise estimate was esteemed at USD 40.0 billion of every 2016 and is relied upon to develop at a CAGR of 5.7% over the gauge time frame. Key drivers affecting the market development are globalization of clinical trials, improvement of latest medications, for instance, customized prescription, expanding advancement in innovation, and boosting interest for CROs to lead clinical trials. CROs enhanced mastery when contrasted with pharma organizations concerning performing clinical trials in wide exhibit of topographies and advancement of medications in particular restorative zones are few components in charge of the developing interest for the CROs in pharmaceutical section.As indicated by BioOutsource, the interest for biosimilars testing is required to increment in the U.S. This is credited to the way that the FDA at long last began tending to the absence of clear direction with respect to biosimilars, particularly how the engineers ought to demonstrate that their medications are like that of the originator item. In January 2015, Hospital submitted one of the biosimilar renditions of Epogen (Epoetin Alfa) and the consequence of the survey in the U.S. is expected from the FDA inside a year.The land dispersion of clinical trials is gradually moving from created countries to rising nations. The increasing expense of clinical trials and trouble in persistent enrolment has driven biopharmaceutical organizations to move towards locales, for example, focal and Eastern Europe, Asia Pacific, Latin America and Middle-East for cost proficiency and snappy patient recruitment. Emerging nations likewise have more prominent malady variety contrasted with west, where conventional infections are developing.The more prominent malady variety among the creating nations encourages biopharmaceutical organizations to perform clinical trials from uncommon sicknesses. Digitization in biomedical research is preparing for development of worldwide clinical trial showcase. Selection of Systems like EDC is additionally helping organizations to better deal with their patient information which eventually lessens the observing expense and help in better patient consistence. Digitization likewise helps in meeting the stringent directions by keeping up tolerant information records which at last aides in decreasing clinical trials process mistakes. He research report on Global Clinical Trials Market 2019 keenly analyses significant features of the industry. The analysis servers market size, latest trends, drivers, threats, and opportunities, also as key market segments.It is supported past data and present market needs. Also, involve distinct business approaches accepted by the choice makers. That intensifies growth and makes a remarkable stand in the industry. The Clinical Trials market will grow with a big CAGR between 2019 to 2028. The report segregates the entire market on the idea of key players, geographical areas, and segments.
Increasing demand for brand spanking new medical equipment’s and medicines among end users, including growing investment for research and development activities for development of effective medicines are major factors driving growth of the global clinical trials market. In addition, increasing number of individuals suffering from chronic diseases as well as changing conditions and nature of certain types of chronic diseases is another factor anticipated to support growth of the worldwide clinical trials market to significant extent.
The study includes basic information about the product such as Clinical Trials scope, segmentation, outlook.
Work is practiced through organization with people there is an increasing interest and awareness in exploring clinical development for rare disease therapies, significant challenges remain in ensuring successful and sustainable clinical trials key topics addressed will include patient identification, recruitment and retention, novel study design and approaches, patient preference studies, preclinical collaborations and evolving technologies to enable adaptive trials. Patient-driven progress will also be highlighted through discussions of changes in the drug development paradigm.
CRO’s, Pharmacy professionals, Association chiefs and Pharma Business people. Professors, Students and to provide an international forum for the spread of original research results, new ideas and practical development experiences which concentrate on both theory and practices, CEO's and Scientists, R and D Professionals and many of them are currently working in this field. Clinical trials, research studies performed on humans, are designed to gain specific information about biomedical interventions such as vaccines, treatments and drugs in order to prove the safety and efficacy of the products. Because of globalization, clinical trials have led to an increase in investment and development of new products in growing countries, resulting in a positive impact on the market. According to an article published recently by Pfizer, the company has three CROs working with it to enhance its product portfolio and drive innovation.
The clinical trials market is projected to arrive at USD 1.76 billion by 2025, from USD 1.04 billion in 2017 growing at a CAGR of 6.7 percent from 2018 to 2025. An upcoming market report contains data for historic year 2016, and the base year of calculation is 2017. Data Bridge Market Research has announced the availability of Clinical Trials Market Outlook to 2025. The global industry report, which covers key players such as PAREXEL, LabCorp, ICON plc, Novo Nordisk, and Covance, provides insights, market dimensions and evaluations for the period from 2018 to 2025. According to Data Bridge, the global clinical trials market is highly fragmented. Data bridge also analyzed major drivers in the market in order to produce the report. They include demand for clinical trials in emerging markets, high R and D spending of the pharmaceutical industry, increasing prevalence of diseases, focus on rare diseases and multiple orphan drugs in pipeline, lack of skilled clinical research workforce, regulatory quality in emerging markets and stringent regulations for patient enrollment. The global clinical trials market is segmented based on phase, design and geographical segments, Data bridge explained. On the basis of phase, the market is further classified into Phase I, Phase II, Phase III and Phase IV. On the basis of design, the market is segmented into treatment studies and observational studies. Treatment studies are further sub-segmented into randomized control trial, adaptive clinical trial and non-randomized control trial. The observational studies segment is further sub-segmented into cohort study, case control study, cross sectional study and ecological study. Data collection and base year analysis are performed using data collection modules with large sample sizes. The market data is analyzed and forecast utilizing market statistical and coherent models. In addition, market share analysis and key trend analysis are critical factors in the market report.
Published: 26-Dec-2020
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.