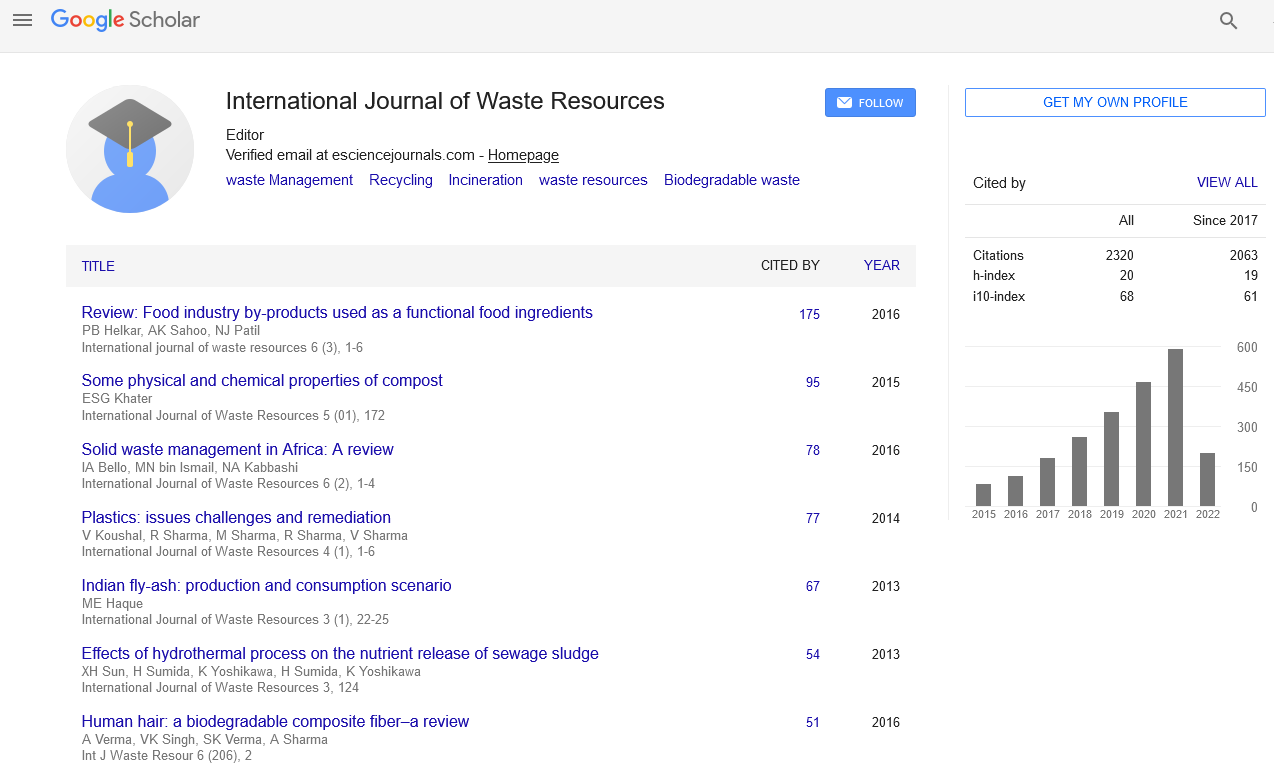
PMC/PubMed Indexed Articles
Indexed In
- Open J Gate
- The Global Impact Factor (GIF)
- Open Archive Initiative
- VieSearch
- International Society of Universal Research in Sciences
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
MA Rahman
MA Rahman
Bangladesh
Publications
-
Research Article
Equilibrium and Kinetic Parameters Determination of Cr(VI) Adsorption by Hogla Leaves (Typha elephantina Roxb.)
Author(s): M Moniruzzaman, MA Rahman, S Aktar and M KhanM Moniruzzaman, MA Rahman, S Aktar and M Khan
Equilibrium and kinetic parameters of Cr(VI) adsorption on Hogla leaves (Typha elephantina Roxb.) were determined in a batch process. Batch adsorption experiments were carried out as a function of pH, adsorbent dosage and initial metal ion concentration. Maximum metal adsorption was found to occur at pH 2.0. The adsorption capacity of studied adsorbent was found to be 30.616 mg/g for initial Cr(VI) concentration of 400 ppm and optimum adsorbent dose of 10 g/L at 25°C. Compared to the Freundlich isotherm model, the Langmuir and Temkin model best fit the experimental data (R2>0.995). Batch adsorption models, based on the assumption of the pseudo firstorder and pseudo second order mechanism were applied to examine the kinetics of the adsorption. The results of this study demonstrated that the pseudo-second order model was more suitable than pseudo-first order model for adsorption .. View More»
DOI: 10.4172/2252-5211.1000301