
Advances in Medical Research
Open Access
ISSN: 2564-8942

ISSN: 2564-8942
Review Article - (2018)Volume 1, Issue 1
Obesity is a prominent health problem in the developed world, and leads to other metabolic diseases. Besides exercise and physical activity, a dietary regimen of fiber-rich food could be a primary solution to overcome obesity. Over the past decades, scientists have been investigating the role of dietary fiber to prevent obesity through innumerable experimental or observational studies. Epidemiological evidences showed that dietary fiber in either soluble or insoluble form helps to reduce weight among overweight or obese adults. This review explores studies that used dietary fiber in different forms to provide a probable conclusion. The objective of this review is to demonstrate the relationship between intake of dietary fiber and its effect on obesity. A comprehensive search for all published academic articles and peer-reviewed up to August 2018 was carried out through a systematic electronic search of several databases. Any interventional, cross-sectional or prospective cohort study from 1980 to 2018 that examined the association between intake of dietary fiber and obesity was included. All the cross-sectional and cohort studies suggested a significant relationship between fiber intake and a reduction in obesity or a classification of being overweight. Within interventional studies, some treatment groups showed no association with fiber supplementation, whereas other studies with longer interventional periods showed a significant association with obesity. Though increased consumption of dietary fiber has an impact on health, it depends on the sources or functionality of the fiber, supplementation or consumption dosage of the fiber, and both the duration of the study and follow-up time points. This suggests that fiber in an edible form or from its mixing with other foods may contribute to prevent the prevalence of obesity.
<Dietary fiber, whole grain, obesity, weight loss
Obesity is defined as a condition of excessive fat accumulation in the body that leads to impaired health conditions. In recent years, the prevalence of central obesity is increasing, whereas it is demonstrated that other non-communicable diseases subsequently occur from central obesity. According to past research, it was revealed that low physical activity, excessive intake of high energy foods, poor consumption of fruits and vegetables, and smoking and drinking alcohol all promote obesity [1-4]. From a prospective observational study with children, it was evidenced that sedentary lifestyles, especially watching television, is becoming a risk factor of obesity [5]. Moreover, excessive fat accumulation in the body leads to a failure to regulate normal physiological processes, and increases the risk of chronic diseases like diabetes [6], certain cancers [7], cardiovascular diseases, hepatic diseases [8], gallstones [9], and gastrointestinal disturbances [10].
In this way, the prevalence of obesity is becoming major public health issue in the modern world.
The European Association for the Study of Obesity declared that obesity was the fifth leading cause of death, whilst 41% of cancer cases were associated with being overweight and obese. The World Health Organization (WHO) set the cutoff point to measure obesity at a body mass index (BMI) of more than or equal to 30 [11-35]. In 1978 it was inferred that dietary fiber (DF) could be a probable solution to prevent obesity [11]. Dietary fiber has been defined by researchers in a variety of ways. The Codex Alimentarius Commission (CAC) in 2009, defined DF as carbohydrate (CHO) polymers (including lignin and/or other compounds associated with polysaccharide from plant cell walls) with ten or more monomeric units, that have physiological health benefits demonstrated by generally accepted scientific evidence. Furthermore, DF is not hydrolyzed by the endogenous enzymes in the small intestine of humans, and belongs to the following categories [12]:
■ Edible CHO polymers naturally occurring in the food as consumed
■ CHO polymers from food, raw material by physical, enzymatic or chemical means
■ Synthetic CHO polymers
Over the past decades it has been recognized that there is a considerable positive effect of DF intake on human health. According to the European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies, 25 g/day of DF intake is adequate for an adult [13]. DF has some unique characteristics which help to reduce mortality and morbidity caused by chronic degenerative diseases [14]. Based on its digestibility in the gastrointestinal tract, DF is classified into two basic groups. The first group is mostly of polysaccharides, that are extracted from plant cell walls, are easily hydrolyzed by enzymatic reactions and absorbed in the small intestine. This group is also known as soluble fibers or soluble fiber polysaccharides such as pectin, obtained from fruits peel [15]. The second group is composed of complex carbohydrates such as cellulose, lignin and pectin which are resistant to digestion in the small intestine, and undergo bacterial fermentation in the colon; defined as insoluble fibers [15]. There are several mechanisms through which DF acts in human body and reduces the risk of chronic diseases [15-16].
Soluble fibers usually undergo fermentation to some extent in the large intestine, and produce some shortchain fatty acids which are absorbed and provide up to 2 kcal/g of energy [17-18]. On the other hand, insoluble fibers increase stool size and bowl movement [19,20]. Sources of such soluble fibers include fruits, vegetables, oats, dried beans and peas and whole grain; however, wheat bran is considered as a source of insoluble fibers [19,20]. Moreover, DF has also been classified as a CHO-based fat replacer, and is used in different dairy products, frozen desserts, and baked goods for example [17].
From 1987 to 2017, a wide range of trials had been completed to investigate the interventional use of DF from numerous soluble and insoluble sources, e.g. cereal fiber, fruit fiber, vegetable fiber, fiber from beans, soy fiber, psyllium fiber, cactus fiber, glucomannan, guar gum, and alginate for example; looking to examine subsequent effects on a body weight. However, the results of the past trials varied depending on the fiber sources.
It was mentioned by Burton-Freeman that DF has a role in controlling level of satiety. In this way, it is possible to regulate food intake and reduce the risk of obesity [26]. It has been hypothesized that fiber-rich foods require more chewing time than normal foods [27]. Moreover, soluble fibers absorb more water and create a viscous gel which increases stomach distention [18]. Pereira and Ludwig described how DF helps to maintain body weight through three distinct physiological pathways; intrinsic, hormonal, and colonic effects. Subsequently, also assists to increase satiety levels and influences on fat oxidation, then decreases energy intake, along with lowering body fat content [28-45]. Other review articles support that DF provides low energy and has significant roles in slowing down gastric emptying, creating a feeling of satisfaction, decreasing serum insulin secretion, and reducing food intake. Furthermore, fermentation of fiber produces short-chain fatty acids which modify eating patterns by releasing peptides and gut hormones such as cholecystokinin and glucagon like peptide 1; thus reducing hunger and promoting satiety [21,29,42]. In an animal model experiment using equal quantities (10% w/w) of both soluble and insoluble fibers, it was evident that insoluble fiber has a significant role in controlling body weight compared with soluble fiber [46-70].
The hormone leptin is primarily responsible for regulating appetite and energy expenditure in humans [64]. It has been evident from an experiment using animals that, leptin resistance occurs with obesity, and failed to maintain hunger balance [23].
Another review article suggested a probable relationship between leptin and ghrelin, where obese patients have leptin resistance, and increased sensitivity to ghrelin [24]. Moreover, in a randomized crossover trial, subjects were exposed to liquid carob fiber (derived from Ceratonia silique, rich in insoluble fibers); reporting a significantly lower level of ghrelin compared to the control group (P<0.001) [33] However, it was concluded that any effects of DF on the secretion of leptin or ghrelin are yet to be fully understood [25].
This review included 20 interventional studies, 4 crosssectional studies and 9 cohort studies, each investigating how different types of fiber supplementation affect body weight.
Search strategy
A systematic literature search was conducted using PubMed and the Cochrane Central Register of Controlled Trials (CENTRAL) databases, for related randomized controlled trials up to August 2018. Other journals like, Journal of European Food Safety Authority, the American Journal of Clinical Nutrition, International Journal of Obesity, and the Journal of Nutrition were also searched directly to extract relevant studies. To reveal information on specific recommendations, international organizations were cited including the World Health Organization and European Association for the Study of Obesity. Specific keywords used including dietary fiber, obesity, weight loss, cereal fiber, whole grain, soy fiber, glucomannan, guar gum, alginate, fruit fiber, and vegetable fiber.
Inclusion Criteria
Any case-control, cohort or experimental study with an intervention involving, or an objective to explore the role of DF in governing weight loss was considered for this review. Studies with healthy overweight or obese subjects at any age were primary selection criteria for cohort and cross-sectional studies, whilst studies involving subjects with a BMI ≥20, or a body weight ≥120% of ideal body weight were accepted for experimental studies. Some of the studies measuring physical activity levels, and providing consultation about daily routine or diet, or exercises along with supplementation were also included. Studies used food diaries, dietary recall, food frequency questionnaires and weighted food records as diet assessment tools. For supplementation, fiber could be consumed as soluble or insoluble DF supplement in the form of tablets or pills, or directly from dietary sources. As inclusion criteria, studies that included body weight loss, BMI and waist circumference selected as primary outcomes and percent of body fat, fecal fat excretion were considered as secondary outcome. In addition, final outcomes were compared with baseline data, and then expressed as a significance level of data using P values or 95% confidence intervals, reported with lower and upper limits.
Exclusion criteria in the selection of studies included pregnancy, diabetes, atherosclerosis, hypertension, renal failure, dyslipidemia or other chronic disease subjects. Also, studies were excluded if the dose of the supplement and the outcomes were not mentioned. Studies were also excluded when partial or multiple interventions were used, and those not mentioning, or missing a control group. Finally, studies published in other languages to English were also excluded. This study does not differentiate between races, gender, socio-economic status, duration or follow up nature of the study, or the method of supplementation. Some additional literature was also studied to gather required knowledge about the biochemical and physiological relation between the study topics.
Study inclusion
This review includes 71 records systematically searched from databases as shown in Figure 1. A total of 2786 articles were searched from the electronic search, 2223 were from PubMed and 563 trials were from Cochrane Central. Full text articles identified for the analysis included 369 articles, with initial screening being done by the title and abstract. On the other hand, 298 records were excluded due to not meeting the defined inclusion criteria. In particular, the 71 records selected for this review included literature reviews, experimental studies, and cohort and cross-sectional studies; with 38, 20, 9 and 4 records of each kind, respectively.
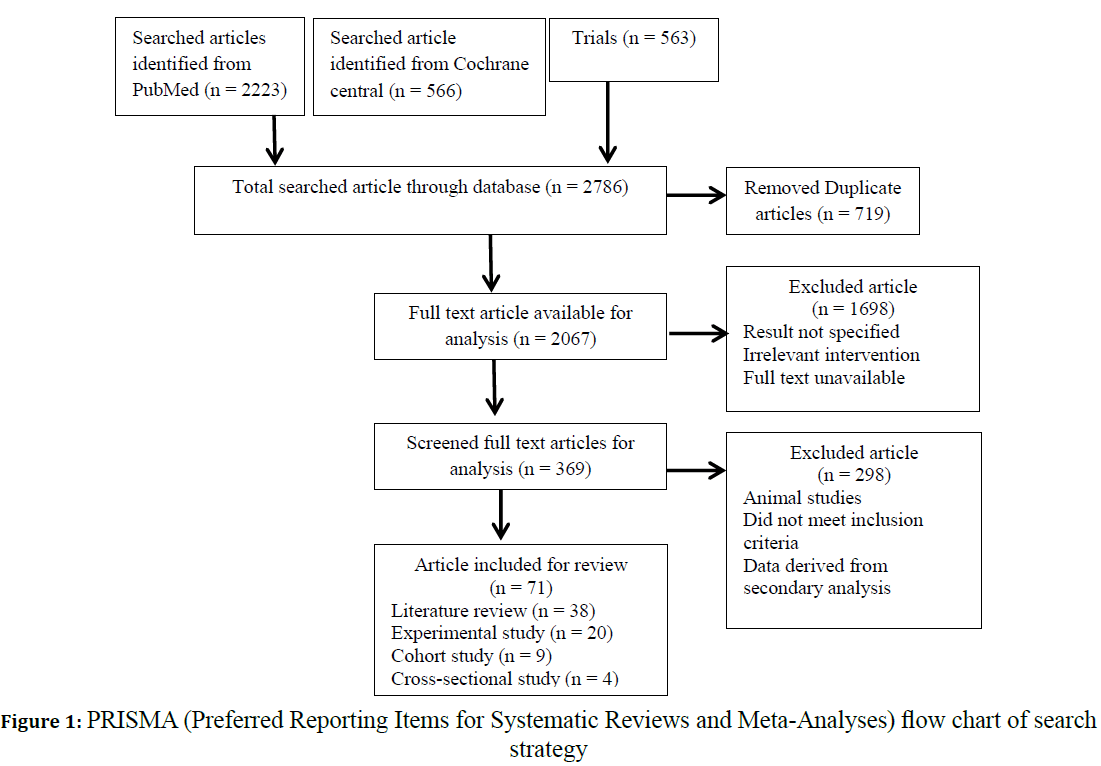
Figure 1: PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart of search
Characteristics of the studies
In this review, 20 interventional studies, 4 crosssectional studies and 9 cohort studies were analyzed to ascertain the association between intake of DF and obesity. Table 1 presents the interventional studies, where 19 were randomized placebo-controlled trials and 1 study was non-randomized trial using low fat plant-based diet along with some dietary guidelines [45-65]. Almost all the experimental studies used different forms of DF, with most results were positively associated, albeit with a few exceptions. Fatahi et al. proposed that whole grain fiber reduced more weight than fruits and vegetables fiber (P = 0.03). However, whole grain with a pulsed intervention program showed no effect on weight loss, but was inversely associated with waist circumference [61]. In 1987, one study compared two groups separately with the effect of grain, citrus fiber and vegetable, and grain and citrus fiber. Although both groups reduced significant amount of weight, the citrus fiber group reduced weight by more (7.0 kg) than the vegetable fiber group (6.2 kg) [65-70]. Dietary fiber in the form of soy fiber or a soybased diet also showed an inverse association in two randomized controlled trials [45,58]. Two studies evidenced that DF along with calorie restriction (low CHO) diet adjunct to exercise-induced significant weight reduction among the treatment group [35,53]. In 2008, Sartorelli et al. implemented a randomized clinical trial with 80 overweight subjects and provided a prescribed diet in the treatment group, reporting that dark colored fruits and vegetables might play a significant role in reducing obesity. A few interventions were performed with functional fiber such as Litramine IQP G-002AS (derived from Opuntia ficus-indica), and PolyGlycoplex and psyllium fiber. PolyGlycoplex in granule form supplemented in a 12 g/day dose showed a reduction in BMI and body weight (P<0.01) compared with a soft gel form [51]. Furthermore, sodium alginate fiber at a 15 g/day dose in conjunction to an energy restricted diet or gum arabic supplementation at 30 g/ day also reduced body weight and fat in the treatment group compared to a placebo [59-62].
| Author, Year | Study Design |
Study Size (M/F); Age (year); BMI(kg/m2); Duration |
Diet Assessment Tool | Exposure | Results |
| Uebelhack et al., 2014 | Double-blind, randomized, placebo-controlled,crossover single center study | 20 healthy subject; 18 to 60; 20 to 30; 45 days |
FD | Cactus fiber tablet | Increase fecal fat excretion compared to placebo 15.79% vs. 4.56% which takes into account the wt loss effect (P <0.001). |
| Grube et al., 2013 | Double-blind, randomized, placebo-controlled, clinical investigation | 123 (30/93); 18 to 60; 25 to 35; 12 weeks | SD | Natural fiber complex Litramine IQP G-002AS |
Body wt loss in treatment group was more in compare to placebo group (Difference 2.4 kg: 3.8 kg vs. 1.4 kg) (P <0.001). |
| Melanson et al., 2006 | Randomized, controlled, two-phase clinical trial |
134 obese adults; 18 to 70; 27 to 35; 24 weeks | 3-day food records |
Hypocaloric diet, fiber rich whole grain cereal, exercise | Both hypocaloric diet plus exercise with whole grain consumption (5.7±0.7 kg) and diet and exercise (6.2±0.7 kg) reduces more body wt than exercise only (1.75±0.62 kg) group (P <0.001). |
| Hypocaloric diet and exercise | |||||
| Exercise noly | |||||
| Turner et al., 2013 | Randomized, controlled trial | 20 (18/2); 18 to 70; 27 to 35; 6 weeks | 3-day food diaries | Beans (29.10 g/day) | Both bean diet (1.62 ±1.26 kg) and SHF diet (1.08±1.70 kg) has equal effects on changes in wt reduction (P <0.001). |
| SHF diet (Fruits, vegetables, WG ) (28.85 g/day) | |||||
| Lambert et al., 2017 | Randomized, double-blind, placebo-controlled study | 50 (9/41) overweight and obese; 18 to 70; 25 to 38; 12 weeks | 3-day weighted FR | Wafers containing 15 g of pea fiber/day | Wt loss was -0.87 ± 0.37 kg (P = 0.022) and fat mass was reduced by -0.74 ± 0.26 kg (P = 0.014). BMI also reduced slightly (P = 0.025) |
| Wafers with no pea fiber | Body wt and fat mass was gained by +0.4 0 ± 0.39 kg (P = 0.022) and +0.42 ± 0.38 kg (P = 0.014) respectively. | ||||
| Hu et al., 2013 | Randomized clinical trial | 39 (17/22) overweight and obese; 19 to 39; 23 to 35; 12 weeks |
7-day food diaries | Soy fiber biscuit, 27.5 g/day | After 12 week BMI (-0.51±0.14) and body wt (-1.39±0.36 kg) significantly reduce (P <0.01). Slight changes observed in WC (-1.75±0.48 cm) (P < 0.05). |
| Control biscuit 3.2 g/day | No significant changes observed in body wt (-0.68±0.32 kg; P = 0.157), BMI (-0.07±0.04; P = 0.129) and WC (0.41±0.15cm; P = 0.937). | ||||
| Keithley et al., 2013 | Randomized, double-blind, placebo-controlled | 47 (7/40) subjects; 18 to 65; 25 to 35; 8 weeks |
3-day FR | Glucomannan 3.99 g/day | No significant difference found in body weight between two groups (−.40 ± .06 and −.43 ± .07 respectively). |
| Placebo capsule | |||||
| Jakše et al., 2017 | Non-randomized interventional trial | 325 (43/282) subjects; mean age 41.2±12; mean BMI 26.5; 10 week | DQ based on food groups | Plant based diet (TDF 40-45 g/day) | 7.3 kg wt reduction found among obese subject (P <0.001). Mean wt change was 1.1 ± 3.6 kg (P= 0.09) and −3.5 ± 5.5 kg (P < 0.001) in control and treatment group. |
| Sartorelli et al., 2008 | Randomized clinical trial | 80 overweight subject; 30 to 65; 24 to 35; 6 months |
FFQ; 75 items | Fruits and vegetable fiber | For 100g of total vegetable and fruit intake 500g and 300g body wt reduces respectively after follow up (P <0.5); and 1.5 kg reduce dark colored vegetables (P <0.05; vegetables [95% CI] = −0.00497 [−0.008, −0.002] and fruits [95% CI] = −0.00290 [−0.005, −0.001]). |
| Solah et al., 2017 | Three-arm, parallel, blind, randomized control trial | 120 (28/92) overweight adult; 25 to 70; 25 to 35; 12 weeks |
4-day mobile food record app | PolyGlycopleX (PGX) soft gels a commercial functional fiber complex 7.6–11.4 g/day. | Non-significant reduction in WC (−0.17 ± 2.92 cm), body wt (0.22 ± 1.61) and BMI (0.07 ± 0.59). |
| PGX granules 12.2 g/day | Significant reduction in WC (-2.5±0.60 cm; P = 0.003), body wt (−1.4±0.10 kg, P < 0.01) and BMI (−0.5 ± 0.10, P < 0.01). | ||||
| RF 12 g/day | Non-significant reduction in WC (−1.3 ± 1.0 cm), body wt (−0.03 ± 0.58) and BMI (0.01 ± 0.20). | ||||
| Tonstad et al., 2014 | Prospective, randomized clinical trial | 173 (45/128)subjects; ≥18; 30 to 44; 16 weeks |
FD | High fiber bean rich diet | Both diet reduces BMI and body wt in two groups [difference, 1.1 kg, 95% confidence interval (CI) = ─2.6 to─0.5; P = 0.2]. |
| Low carbohydrate diet | |||||
| Birketvedt et al., 2005 | Randomized double blind placebo-controlled study | 176 subject; 30 to -60; >25 to <30; 5 weeks |
SQ | Glucomannan | Significant wt reduces after administration in compare to placebo for three groups approximately 0.8 kg/week, (3.8±0.9, 4.4±2.0, 4.1±0.6 respectively) but no individual changes observed (P <0.001). |
| Glucomannan, guar gum | |||||
| Glucomannan, guar gum and alginate | |||||
| Rössner et al., 1987 | Randomized, double-blind parallel study | 54 female subjects; 18 to 60; body wt ≥120% of IBW; 2 months | 24 hour DR | Fiber tablet (grain and citrus fiber) | Mean wt reduction in fiber group was higher (7 kg) than placebo group (6 kg) (P <0.05). |
| Placebo tablet (corn starch and sucrose) | |||||
| 41 female subjects; 18 to 60; body wt ≥120% of IBW; 3 months | Fiber tablet (vegetable, grain and citrus fiber) | Mean wt reduction in fiber group was 6.2 kg, that is higher than placebo group (4.1 kg) (P <0.05). | |||
| Placebo tablet (corn starch and sucrose) | |||||
| Salas-Salvadó et al., 2008 | Parallel, double-blind, randomized placebo-controlled, multi-centered clinical trial | 166 adults; 18 to 70; 27 to <35; 16 weeks |
FD | Mixed fiber dose (3 g Plantago ovata husk and 1 g glucomannan) twice a day | Wt losses observed in both fiber group (-4·52±0·56 kg and -4·60±0·55 kg respectively) and with the placebo group (-3·79±0·58 kg) but there was no significant differences between the groups (P = 0.43). |
| Mixed fiber dose thrice a day | |||||
| Placebo sachets(3 g microcrystalline cellulose) | |||||
| Pal et al., 2016 | Randomized, double-blind, parallel design study | 127 (54/73) overweight adult; mean age 49.2; 25 to 47; 52 weeks |
3-day food and drink diary | PolyGlycopleX (PGX, NSP complex) 15 g/day | Weight reduction in the PGX group was −2.8% and −1.5% for the PSY group compared to control after 12 months supplementation WC and body fat significantly lower in compare to control group (P <0.05). |
| Psyllium fiber (PSY) 15 g/day | |||||
| RF 15 g/day (Placebo) | |||||
| Allison et al., 2003 | Randomized controlled clinical trial | 100 (20/80)obese subject; 35 to 65; 28 to ≤41; 12 weeks |
SSI | Soy based meal diet (Scan Diet meal-replacement formula) | Mean wt loss was 7.1 kg (95% CI 5.4, 8.8; P = 0.001). |
| 1200 kcal exchange system diet | Mean wt loss 2.9 kg (95% CI 1.8, 4.0; P <0.0001). | ||||
| Georg Jensen, et al., 2012 | Randomized, double-blind, parallel design study | 80 (26/54) obese subject; 20 to 55; 30 to 45; 12 weeks |
FD | Sodium alginate fiber 15 g/day | Significant wt loss was observed in alginate group than control group (-1.7±0.5 kg, P =0.031). |
| Placebo (maltodextrin and sucrose) | |||||
| Fatahi et al., 2018 | Randomized parallel controlled feeding trial | 75 female adults; 18 to 50; ≥25; 10 weeks |
3-day FR | WG | WG group significantly reduces more wt (P =0.03) and WC (P=0.001) in compare to other two groups. |
| Fruits and vegetables | |||||
| WG, fruits and vegetables | |||||
| Venn et al., 2010 | Randomized controlled study | 108 overweight subject; ≥40; ≥28; 18 months |
3-day weighted FR | WG & pulse | No different observed between treatment and control group wt loss (P >0.05); but significant reduction in WC observed in exposure group (-2.8 cm; 95% CI: -0.4, -5.1). |
| Control diet of NFHNZ | |||||
| Babiker et al., 2012 | Two-arm randomized, placebo controlled, double-blind study | 120 female subject; ≥17; mean BMI 26.5±4.6; 6 weeks |
NA | Gum Arabic 30 g/day | Slight reduction of BMI (95% CI: 0.17 to 0.47; P <0.0001) and body fat percentage (95% CI: 1.54 to 283; P <0.0001). |
| Placebo (pectin) 5 g/day | Slight increase in BMI (95% CI: -0.16 to 0.02; p = 0.132) and percentage of body fat (95% CI: -1.44 to −0.20; P = 0.010). |
FFQ; Food Frequency Questionnaire, FD; Food Diary, SD; Subject diary, wt; weight, DQ; Dietary Questionnaire, SHF; Standard High Fiber, TDF; Total Dietary Fiber, RF; Rice Flour, SQ; Standardized Questionnaire, DR; Dietary Recall, IBW; Ideal Body Weight, NSP; Non-Starch Polysaccharide, SSI; Standardized Structured Interview, FR; Food Record, NHFNZ; National Heart Foundation of New Zealand, WG; Whole Grain, WC; Waist Circumference, NA; Not Applicable.
Four cross-sectional studies with more than 20,000 subjects examined the role of whole grain compared with refined grain to reduce obesity (Table 2). The National Health and Nutrition Examination Survey (NHANES) 1999 to 2004 was cross-sectioned between the consumption of whole grain and cereal fiber; reporting that intake of whole grain was inversely associated with obesity.
| Author, year |
Study design | Study size (M/F); Age | Diet assessment tool | Exposure | Results |
|---|---|---|---|---|---|
| Newby et al., 2007 | Baltimore Longitudinal Study on Aging | 1516 subject; 27 to 88 year | 7-day dietary records | WG, CF, RG | WG and CF intake was inversely associated with BMI (P <0.0001), weight, (P =0.0004), WC (P <0.0001). For WG consumption a significant difference observed with subjects in the lowest quintal (Q1) to the highest quintal (Q2) for BMI (Q1, 25.5; Q5, 24.8; P < 0.0001), wt (Q1, 75.0 kg; Q5, 72.6 kg; P= 0.004) and smaller WC (Q1, 87.4 cm, Q5, 85.0 cm; P =0.002). |
| Lutsey et al., 2007 | Multi-Ethnic Study of Atherosclerosis | 5496 subjects; 45 to 84 year | FFQ, 127 food items | WG | WG intake was inversely associated with BMI (mean differences between highest and lowest quintal was 0·6 kg/m2, P <0.0001). |
| McKeown et al., 2010 | Framingham Heart Study | 2834 (1434/1400)subjects; 32 to 83 year | Semi quantitative FFQ, 126 food items | WG | Inverse association between WG intake with WC (97.0 compared with 93.7 cm in the lowest compared with highest quintile category, P <0.001). |
| RG | Positive association between RG intake with WC (95.9 compared with 97.3 cm, P = 0.06). | ||||
| O'Neil et al., 2010 | National Health and Nutrition Examination Survey1999 to 2004 | 7039 for 19 to 50 year and 6237 subjects for ≥51 year | 24 hour dietary recall | WG | WG intake was inversely associated with BMI and WC (P =0.04) among 19 to 50 year; for age ≥51 year BMI (P =0.03) and WC (P =0.01). |
| Total CF | No association observed with obesity. |
FFQ; Food Frequency Questionnaire, WG; Whole Grain, CF; Cereal Fiber, RG; Refined Grain, WC; Waist Circumference.
Table 2: Results of Cross-sectional study
Nine prospective cohort studies (Table 3) including 855,974 participants showed a significant relationship between DF intake and a change in body weight. Ludwig et al. conducted a population-based prospective cohort study for 10 years, concluding that DF is independently and inversely related to weight reduction [36]. A considerable number of studies had conducted examining the role of whole grain intake and weight reduction. However, Rautiainen et al. in 2015 postulated that fruit fiber intake is more strongly inversely associated with weight loss compared with vegetable fiber [48].
| Author; Year |
Study name | Study size (M/F); Age; Follow up period |
Diet Assessment tool | Exposure | Results |
|---|---|---|---|---|---|
| Koh-Banerjee et al., 2004 | The Health Professionals Follow-up Study | 27082 subjects; 40 to75; 8 years |
Semi quantitative FFQ, 250 food items |
WG, FF, VF | 20 g/day increase in DF wt gain reduced by 1.18 kg (whole grain, P <0.0001) & 2.51 kg (fruit fiber, P <0.001). |
| Ludwig et al., 1999 | The Coronary Artery Risk Development in Young Adults Study | 2909 healthy adults, 18 to 30; 10 years |
Interviewer administered quantitative FFQ; 700 foods | TDF | Fiber consumption inversely associated with wt gain (P <0.001). |
| Liu et al., 2003 | Nurses’ Health Study | 740191 female subjects; 38 to 63; 12 years |
Validated FFQ; 61 item | RG | Wt gain inversely associated with WG consumption rather than RG ( P <0.0001). |
| WG | |||||
| Van et al., 2009 | Netherlands Cohort Study | 4237 (2078/2159) subjects; 55 to 69; 5 years |
Semi-quantitative FFQ; 150 food items |
BB | No significant association found between intake and obesity. |
| WG | Inverse association found with intake and wt gain (P <0.01). | ||||
| Steffen et al., 2003 | Minneapolis Public School Students Study | 285 (155/130) healthy adolescents; mean age 13; 2 years | Two FFQ, 127 food items | WG, RG | WG intake was inversely associated with BMI (P = 0.01) than RG. |
| Lindström et al., 2006 | Finnish Diabetes Prevention Study | 522 (172/350) overweight middle aged adults; 40 to 64; 3years |
3 day food record | High fiber, Low fat | 3.1 kg wt reduces (95% CI 2.3–3.9) (P = 0.001). |
| Low fiber, high fat | 0.7 kg wt loss (95% CI−1.7 to +0.1). | ||||
| Tucker & Thomas: 2009 | NS (covering two metropolitan areas in Mountain West region) | 252 women; mean age 40.1; 20 months | 7day weighted food records | TDF | Weight decreased by 0.25 kg (P =0.0061) and fat decreased by 0.25 percentage point (P =0.0052) for 1 g increase in total fiber consumption. |
| Du et al., 2009 | DiOGenes (Diet, Obesity, and Genes) project | 89432 (37125/52307) European subjects; 20 to 78; 6.5years |
Country specific FFQ | Cereal fiber | Inversely associated with weight change (P = 0.01) |
| Fruits and vegetables fiber | No strong association (P = 0.05) | ||||
| Rautiainen et al., 2015 | Women's Health Study | 18,146 women; age ≥45; 17 years |
FFQ, 131 food items | FF | Strong inverse association found between fruit intake and BMI (HR: 0.82; 95% CI: 0.71, 0.94). |
| VF | Associated with wt gain (P = 0.02) |
FFQ; Food Frequency Questionnaire, wt; weight, WG; Whole Grain, FF; Fruit Fiber, VF; Vegetable Fiber, RG; Refined Grain, TDF; Total Dietary Fiber, FR; Food Record, BB; Brown Bread
Table 3: Results of Prospective cohort study
In total, 33 studies were examined, using different types and sources of DF and several functional fibers. During the trial period, subjects were guided with dietary advice to drink a minimum level of water or skim milk before taking the supplements [45, 46, 51, 54, 59]. To minimize potential discomforts, fiber doses in interventional studies were increased gradually throughout the supplementary period; whilst results were subsequently compared with baseline characteristics. It was evident from the reviewed trial that, pea fiber was a good source of DF, comprising of 8% soluble fiber from 92% total fiber; where moderate doses of yellow pea fiber (15 g/day) significantly helped reduce body fat among overweight or obese subjects [44]. It was hypothesized that glucomannan, a fermentable water soluble DF (known as konjac) helped to reduce body weight by inducing bulking properties. However, an 8-week trial of glucomannan ingestion alone showed no significant changes in weight compared with a placebo [46]. Also, a 5-week trial of glucomannan in combination with guar gum and alginate resulted in a reduction in weight in the treatment group compared with a placebo [54].
In terms of palatability and appetite, fiber-rich foods are less palatable and create a feeling of fullness after having a low amount, whilst foods and hunger ratings are inversely related with time [55,69] There is substantial evidence that whole grains are associated with lowering the percentage of abdominal adiposity compared with refined grain [70]. This can be further elaborated from a recent cross-sectional study with middle-aged and elderly Japanese people; the WASEDA'S Health Study. The results revealed that a healthy dietary pattern was inversely related to waist circumference and visceral fat [4]. Kehlet et al. compared hunger, fullness, satiety and palatability levels between four test meals, demonstrating that the addition of fiber to meals leads to less palatability, as well as increased satiety and fullness perceptions, and mitigated hunger. Overall, DF could be a probable solution to alleviate overweight or obesity [18,71].
Mattes in 2002 hypothesized, based on a cereal intervention involving a volumetric diet and fiber-rich foods, that adopting such a diet will reduce energy intake as well as fat mass [39]. In a study named The New Dietary Interventions to Enhance the Treatments for weight loss (New DIETs), researchers investigated the effects of a weight loss intervention using a plantbased diet composed of fruits, vegetables, whole grains, and legumes/beans; providing evidence that a plantbased diet could be a short-term healthy approach to reduce weight [41].
This study examined probable relationships between DF and obesity, by analyzing experimental, crosssectional and cohort studies. Heterogeneous results were identified, even when using the same protocol, or diet assessment tools. Therefore, variations in results may be due to subjective anthropometric and physiological variations, and the period of follow up, for example. In short, research on the use of DF may have wide-ranging opportunities relating to the treatment of obesity. However, further research is required to investigate stronger associations between DF intake and obesity.
All work has been carried by the authors. There was no conflict of interests. Also, we would like to thank Dr. Llion Roberts, Lecturer of Human Physiology, School of Allied Health Sciences, Griffith University, for English editing.
None to report.
Citation: Ruhee RT, Suzuki K (2018) Dietary Fiber and its Effect on Obesity, Adv Med Res 1:1. doi:10.12715/amr.2018.1.3
Received: 27-Aug-2018 Accepted: 12-Sep-2018 Published: 20-Sep-2018
Copyright: © 2018 Ruhee RT. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.