PMC/PubMed Indexed Articles
Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
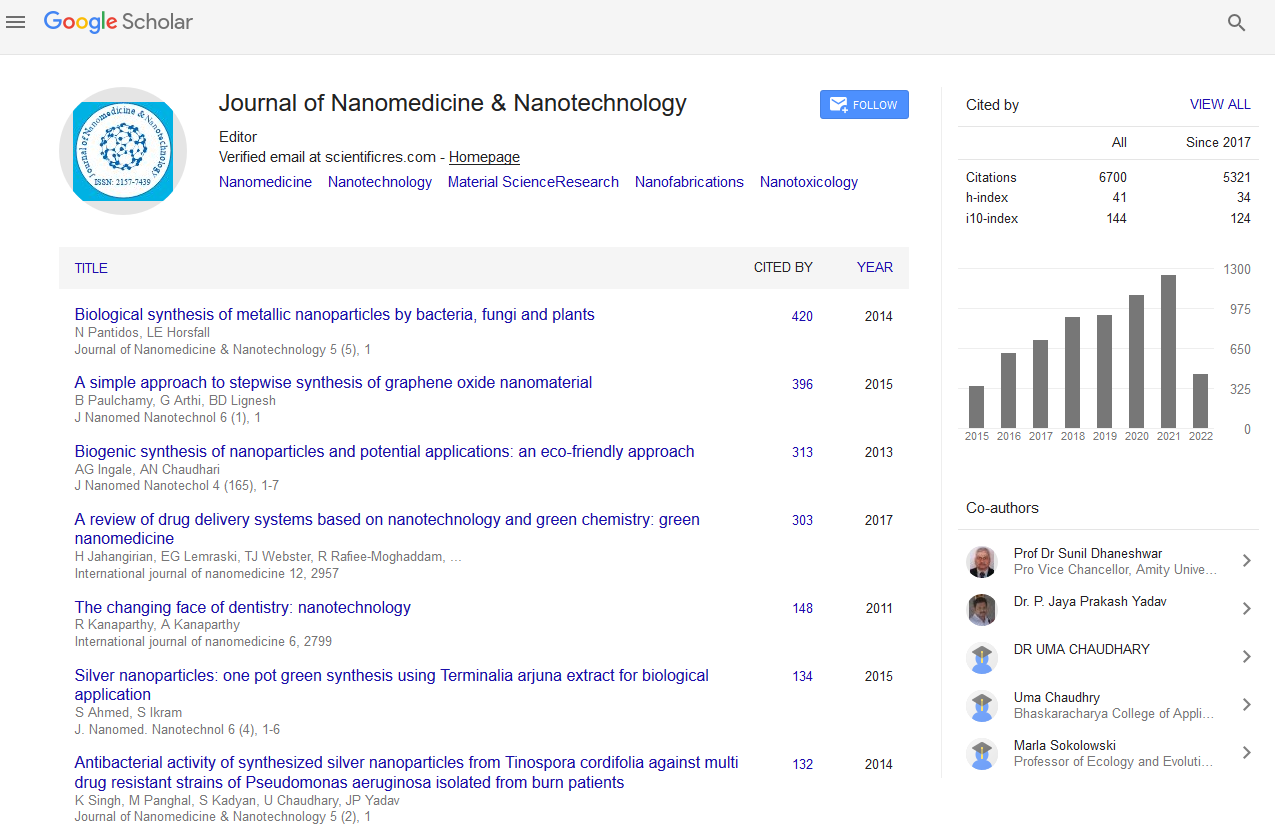
Kinetic investigation of the catalytic oxidation of dibenzothiophene to dibenzothiophene-sulfone using TiO2
World Congress on Petrochemistry and Chemical Engineering
November 18-20, 2013 Hilton San Antonio Airport, TX, USA
J. H. Leal and J. G. Parsons
Posters: J Pet Environ Biotechnol
Abstract:
The oxidation of dibenzothiophene (DBT) to dibenzothiophene-sulfone has been considered as an alternate method to remove sulfur from crude oil. This study demonstrates that the oxidative desulfurization (ODS) of DBT to DBT-sulfone occurs using TiO 2 as a catalyst. The rutile polymorph of TiO 2 was synthesized and characterized using XRD to confirm phase and to determine particle size. In addition, SEM micrographs were taken of the particles to observe morphology of the material. The catalytic properties of the rutile polymorph of TiO 2 were investigated from 110 o C to 140 o C. Aliquots of 1.0 mL were taken at 30 minute intervals over four hours to determine the reaction kinetics. The oxidation reaction was performed by refluxing TiO 2 and DBT in decahydronapthalene while flowing O 2 through the reaction mixture. The disappearance of DBT was followed using gas chromatography-mass spectroscopy. In addition the identity of the reaction product was confirmed with FTIR, RAMAN, and XRD. Gas chromatography-mass spectroscopy results showed greater than 95% conversion of DBT to DBT-sulfone within 90 minutes at temperatures greater than 120 o C. Kinetic studies showed the reaction followed first order kinetics. In addition, from Arrhenius plots the activation energy for the oxidative desulfurization of DBT to DBT-sulfone was determined to be 43.6KJ/mol.
Biography :
Juan Leal has completed his B.S. in chemistry and is completing his M.S. in chemistry. Juan has published one manuscript in a reputable journal as an undergraduate