Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 9, Issue 2
Validating Effect of Sulodexide in Treatment of Claudication Pain and Chronic Limb-Threatening Ischemia
Lubos Kubicek1,2*, Martin Dvorak1,2 and Robert Staffa1,22Masaryk University, Brno, Czech Republic
Received: 19-Feb-2021 Published: 12-Mar-2021, DOI: 10.35248/2329-6925.21.9.409
Abstract
Background: Peripheral arterial disease is a chronical disease which can lead to a sever quality of life reduction and in certain cases there is no indication or even possibility for revascularization and these patients are reliant on conservative therapy such as Sulodexide medication.
Objective: In the retrospective study we evaluated effect of newly administrated Sulodexide therapy for patients with peripheral arterial disease both in cases of claudication pain and patients with chronic limb-threatening ischemia (CLTI).
Methods: During a three year period we started Sulodexide therapy in 34 claudication and 38 CLTI cases with no contemporary revascularization procedure. Patients were followed for 4 and 8 months with claudication interval, Rutherford classification and CLTI evaluation.
Results: We observed overall positive effect of Sulodexide in both groups. In claudication group there was a prolongation of average pain-free walking distance (PFWD) from 144 m at the baseline to 376 m at 4 months and 430 m at 8 months. In the second group all 38 patients started with CLTI symptoms, after 4 months 6 patients remained with CLTI and after 8 months 3 more had recurrence of CLTI.
Conclusion: We observed a significant improvement both in PFWD in claudication sub-group and even in a clinical status of majority of the patients in CLTI sub-group. This effect of Sulodexide on CLTI patients was not yet thoroughly described in any available literature. According to our observations, Sulodexide seem to be a potent auxiliary therapy for patient with peripheral arterial disease even in the case CLTI.
Keywords
Peripheral arterial disease; Claudication; Ischemic wound; Critical limb ischemia; Sulodexide; Conservative treatment
Abbrevations
ABI: Ankle-Brachial Index; CLTI: Chronic Limb-Threatening Ischemia; PAD: Peripheral Arterial Disease; PAI-1: Plasminogen Activator Inhibitor-1; PFWD: Pain-Free Walking Distance; tPA: tissue Plasminogen Activator
Introduction
Peripheral arterial disease (PAD) is one of the most concerning disease of this era, mostly because it can be caused by a modern way of living in developed countries [1]. Risk factors of PAD are hypertension, diabetes mellitus, hyperlipidemia, smoking, fatty diet and genetic factors [2]. Prevalence of PAD is rising each decade as the population in these countries progressively ages. At the day the prevalence of symptomatic PAD is stated in literal sources up to 6% of population older than 60 years with majority of men [3]. Symptomatic PAD is not only associated with higher risk of coronary and cerebrovascular morbidity and mortality [4] but, what is the most important from a patients point of view, it is significantly decreasing the quality of life of these patients [5] by limiting their daily activities, disables them in work process and in the worst case leading to critical limb ischemia (termed by the last nomenclature as chronic limb threatening ischemia-CLTI) presented by a rest pain and chronic wounds with all the severe consequences [6]. Prevalence of critical limb ischemia is still at significant level between 0.25 and 0.3% of the same population [7].
First and the most specific symptom of PAD is claudication pain, which limits patient in walking by extend of atherosclerotic process damaging the lower limb arteries [8]. Claudication pain, if PAD is not treated, progresses in time as the atherosclerosis is involving larger portion of lower limb arteries and in more severe degree causing an arterial occlusion. Progressing claudication pain can lead to disability of the patient and in stage of CLTI to a rest pain and ischemic skin wounds; this stage is associated with very high morbidity and mortality [9]. One year mortality of patients with CLTI ranges between 10 and 40% and if there is no revascularization performed up to 40% of the patients end up with high amputation to six months from the point of first signs of CLTI [7].
After PAD diagnosed (clinical symptoms, imaging methods and functional tests e.g. ABI) the next course of actions is determined by the degree of clinical symptoms (e.g. Rutherford or Fontaine classification) and by the extent of atherosclerotic process [10]. Essential treatment for PAD patients is elimination of risk factors [2] (mostly smoking), regular exercise and arterial revascularization which can be performed both by endovascular procedures or open surgery [11]. Nevertheless, there are certain situations when these procedures are not indicated or technically unfeasible. Most of these patients are situated on both exactly opposite ends of PAD spectrum-first there are a patients with intermittent claudication with long Pain-Free Walking Distance (PFWD) which do not meet indication criteria for intervention [10] but even this degree of symptoms is limiting their usual daily or working activities (e.g. watchman or postman). Second group of these patients is much worse in nature; these are the patients with exhausted options of revascularizations or with such a severe degree of occlusive process prohibiting any revascularization procedure [12]. Treatment of these two groups of patients should consist of regular exercise by walking [13] (which is however often very limited by the presence of the skin wound), intensive local therapy of skin wounds in cases of CLTI and also of supportive medication [14]. There are several drugs used for medical (or also “conservative”) therapy of PAD, all clinically tested with more or less controversial outcomes. Promising results are mostly mentioned regarding the use of Sulodexide in patients with claudication pain [15-18]. Several randomized trials [19] and meta-analyses [15] were performed to evaluate its effect on claudication pain as well as other factors like serum level of fibrinogen, blood and plasma viscosities and serum lipid panel. In accordance with these sources we observed in our center through the last three years of higher rate of Sulodexide usage its overall positive effect in conservative and auxiliary treatment of both groups of patients with claudication pain and patients with CLTI.
Sulodexide is a standardized extractive glycosaminoglycan containing 80% “fast moving” heparin and 20% dermatansulphate, which has a high affinity for antithrombin-III and heparin cofactor-II, it also activate lipoprotein lipase (LPL) and has an antithrombotic and fibrinolytic activity through the activation of tPA and inhibition of PAI-1 [20]. All these Sulodexid activities were demonstrated after both intravenous and oral administration [21].
Main goal of our retrospective study was to evaluate the effect of Sulodexide as conservative treatment for PAD patients both with claudication pain (Rutherford 1-3) and with CLTI (Rutherford 4-6).
Materials and Methods
The retrospective study of patient documentations in our center was performed seeking all patients with newly administrated Sulodexide medication. This study covered a period of three years between January 2017 and December 2019. All patients signed the consent with use of their clinical data for research and educative purposes at the beginning of the treatment; this is the standard procedure in our center. As we wanted to evaluate the effect of Sulodexide only, we excluded all patients after contemporary revascularization procedure which received Sulodexide as an auxiliary medication after the revascularization. At the end we got two groups of patients-first is a group of patients with claudication pain not meeting the criteria for revascularization and second is a group of patients with CLTI with no more possibilities for radical treatment.
Basal clinical data before the start of Sulodexide treatment were gathered for both groups as well as PAD specific data which were followed through patient documentation. All patients were stratified by Rutherford classification [22] Rutherford 1-3 for the first group and Rutherford 4-6 for the second group.
In the first group we followed change in patient claudication status with PFWD as the main parameter. The claudication status was followed in the second group as well but the main parameter for this group was the presence of critical ischemia represented by a rest pain or presence of ischemic skin wound. CLTI parameters were described in “yes” or “no” way, with elimination of rest pain or complete healing of the wound considered as a treatment success. This simplification in skin wound description was required because of a huge variability in wound characteristics [23] (depth, size, location, portion of necrotic tissue etc.), which could not be properly described and compared between each patient using only a standard outpatient documentation.
In both groups there were two more sub-groups described based on the beginning of Sulodexide therapy-patients starting with intravenous application (10 days infusion therapy) and then continued with oral medication (as is suggested protocol in Summary of Product Characteristics for Vessel Due F®) and the second sub-group of patients starting directly with an oral medication (mostly the patients refusing intravenous application).
Moreover all follow-up data from both groups were divided in another two sub-groups based on the length of follow-up to cover up an effect of Sulodexide through short and long term period of treatment. This division means that the data after 4 months period and 8 months period were used for statistical analysis.
Thus eight sub-groups were created describing a change of PFWD in first main group and CLTI status in second main group, comparing the effect of intravenous and oral Sulodexide administration in short and long term follow-up for both main groups.
All data were statistically tested using software Statistica 12 (StatSoft, Inc., USA) for basic descriptive analysis and comparison of sub-groups. F-Test for parametric data distribution was used to calculate statistical significance (at p level of 0.05) for changes in PFWD, CLTI status and Rutherford classification.
All patients received treatment in accordance with standard of care in our center and in accordance with Summary of Product Characteristics for Sulodexide (Vessel Due F®, Alfasigma S.p.A., Italy) [24]. Sulodexide treatment protocol in our center is as follows: treatment should start with parenteral application (in our center we use solely intravenous application) of 600 LSU (1 ampoule) each day for 10 days and then continue with an oral administration of 1 tablet BID for at least six months (in our center we use 1 tablet BID as standard, no patient had 2 tablets BID). In cases when patients refuse to start the treatment with intra venous administration the treatment is initiated directly with an oral form. Different procedures of drug delivery can lead to different concentrations of Sulodexide in patient’s serum and our goal was to assess if there is any difference in effect of intravenous and oral drug administration. This assessment was based only on clinical status of the patients with no pharmacological tests included, what can be considered a limitation of this study; no pharmacological tests were possible in retrospective settings. Physical training in form of regular walking was strongly suggested to all patients as a part of standard treatment (if not disabled by a wound) [13]. Vigorous controlling of patients diabetes status was advised as well, but this was carried out by their general practitioners or internal physicians with no involvement of our team.
Results
During the three years period the Sulodexid treatment started in 72 cases of patients with PAD. Out of this population 34 cases were patients with claudication pain (Rutherford 1-3) and 38 cases patients with CLTI (Rutherford 4-5). There was no significant difference in number of diabetic patients in both group-38.2% (n=13) for claudication group and 42.10% (n=16) for CLTI group (p=0.938). If we sub-divide the CLTI group to patients with rest pain (Rutherford 4, n=30) and with ischemic wound (Rutherford 5, n=8) the percentage of diabetic patients changes significantly-30.0% for Rutherford 4 and 87.5% for Rutherford 5. Complete list of patients in both groups together with their basic medical characteristics are listed in Appendix.
Majority of the patients in both groups started the treatment as recommended with parenteral (in all our cases intravenous) application, with even bigger portion in CLTI group intravenous application was a start of the treatment for 20 patients (58.8%) in claudication group and for 27 patients (71.0%) in CLTI group. Effect of the treatment was evaluated for each sub-group separately.
Claudication group
At the baseline patients started with an average PFWD of 144 m and baseline Rutherford class 2.62. There were a slight differences for intravenous and oral group with starting PFWD of 71 m and 191 m respectively (p=0.023), meaning that patients with shorter PFWD were more urged to start the treatment with intravenous application and patients with longer PFWD were more often allowed to start treatment directly with oral medication. Both subgroups were followed for 4 months (average 131 days) and 8 months (average 254 days) with PFWD and Rutherford assessed. Altogether the PFWD was extended (from baseline 144 m) to 376 m at 4 months and 430 m at 8 months (Table 1), meaning final extension of PFWD by +286 m in average (2.98 times more than baseline PFWD, p=0.001). Walking distance converted to the Rutherford classification gives overall drop of-0.83 class from baseline 2.62 to 1.88 at 4 months and 1.79 at 8 months (p=0.002) (Table 2).
| Claudication | Baseline | follow-up 131 days (89/161) | follow-up 254 days (244/261) | ||||
|---|---|---|---|---|---|---|---|
| Patients (n) | PFWD (m) | Rutherford | PFWD (m) | Rutherford | PFWD (m) | Rutherford | |
| Total | 34 | 144 | 2.62 | 376 | 1.88 | 430 | 1.79 |
| Intravenous | 20 | 71 | 2.93 | 302 | 2.07 | 362 | 1.93 |
| Oral | 14 | 195 | 2.4 | 428 | 1.75 | 478 | 1.7 |
Table 1: Characteristics of Claudication group as total and in both sub-groups. (All values are the average value of the respective group).
| PFWD improvement (m) | Rutherford improvement | |
|---|---|---|
| Total | (+) 286 | (-) 0.83 |
| Intravenous | (+) 291 | (-) 1.00 |
| Oral | (+) 283 | (-) 0.70 |
The effect of the treatment was more significant in sub-group starting with intravenous application with PFWD extension from 71 m at the baseline to 302 m at 4 month and up to 362 m at 8 months, meaning overall extension of PFWD by +291 m (5.09 times more than baseline PFWD, p<0.001). Rated by Rutherford classification this means a drop of-1.00 class from baseline 2.93 to 2.07 at 4 months and 1.93 at 8 months (p=0.008).
Oral sub-group reaches a longer PFWD in average at the end of follow-up, but there was a significantly higher starting value of 195 m in this sub-group (compared to 71 m for intravenous subgroup), thus the overall effect was smaller than in intravenous group, but still highly significant. There was an extension from 195 m PFWD at the baseline to 428 m at 4 months and to 478 m at 8 months (Table 1). Overall extension of PFWD in oral subgroup was +283 m (2.45 times more than baseline PFWD, p=0.038). Rated by Rutherford classification this means a drop of -0.70 class from baseline 2.40 to 1.75 at 4 months and 1.70 at 8 months, what was also rated statistically significant (p=0.045) (Table 2).
During the follow up there was a worsening of PAD status in one diabetic patient starting in oral group as Rutherford 3, with CLTI in form of ischemic skin wound at 4 months (Rutherford 5) lasting further through whole follow-up. In three more cases there was a shortening of PFWD observed between 4 and 8 month follow-up, but in all three cases the PFWD remained longer than the baseline PFWD of these patients.
Chronic limb-threatening ischemia (CLTI) group
Because it would be confusing to compare claudication pain in cases of patients with CLTI (most of the patients with rest pain and limitation in walking caused by wound) we evaluated the Sulodexide effect on critical ischemia only-meaning if patient was relieved of rest pain or if the ischemic wound was healed, we considered it as a success of the treatment. Changes of Rutherford class were evaluated as well. Again all patients were followed for 4 months (average 101 days) and 8 months (average 233 days).
At the baseline all patients in this group had symptoms of critical limb ischemia with rest pain or non-healing ischemic wound (Figure 1). Most of the patients started with intravenous application of Sulodexide as is recommended by our protocol. But still there were 11 patients (out of total 38) starting the treatment directly with oral medication. These patients refused intravenous application despite of being acknowledged about the importance of more intensified approach in the state of CLTI.
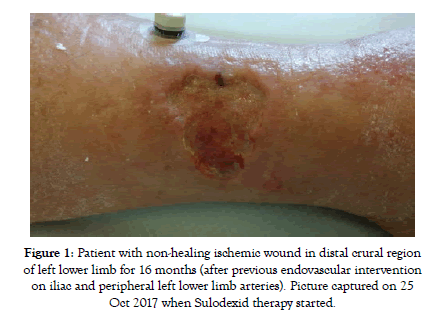
Figure 1: Patient with non-healing ischemic wound in distal crural region of left lower limb for 16 months (after previous endovascular intervention on iliac and peripheral left lower limb arteries). Picture captured on 25 Oct 2017 when Sulodexid therapy started.
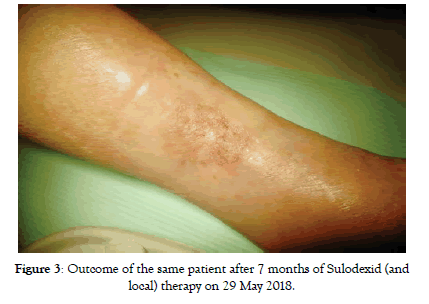
Although we urged patients to intravenous application there was no significant difference between the effects if we compare these two sub-groups. In intravenous group we started with 27 patients with CLTI, at 4 months there were only 4 patients left with CLTI (no treatment effect cases), all other with no rest pain or with the baseline wound healed (Figure 2). These 4 patients remained with CLI till the end of follow-up. Status of 2 more patients worsened between 4 and 8 months follow-up again back to CLTI (Table 3). At the end of follow-up there were 6 patients with CLI and 21 patients with positive treatment effect (equal to 77.7%) (Figure 3). Regarding the Rutherford classification we got the same outcome starting with average Rutherford 4.15 which got improved to 2.15 at 4 months and worsened to 2.37 at 8 months. This means the improvement of-1.78 Rutherford class (p<0.001) (Table 4).
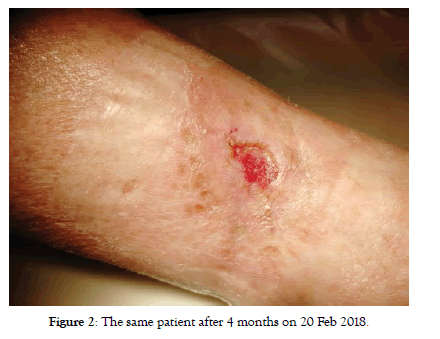
Figure 2: The same patient after 4 months on 20 Feb 2018.
| PFWD improvement (m) | Rutherford improvement | |
|---|---|---|
| Total | (+) 286 | (-) 0.83 |
| Intravenous | (+) 291 | (-) 1.00 |
| Oral | (+) 283 | (-) 0.70 |
Table 3: Characteristics of CLTI group as total and in both sub-groups. Number of CLTI patients means a ratio between patients with CLTI symptoms (rest pain or wound) on the left site and patients without these symptoms after the treatment on the right site, these numbers together form the initial number pf CLTI patients at the baseline. (Rutherford values are the average value of the respective group).
Figure 3: Outcome of the same patient after 7 months of Sulodexid (and local) therapy on 29 May 2018.
Outcomes for oral treatment sub-group are similar, starting with 11 CLTI patients with average Rutherford class 4.36. At 4 months follow-up there were 2 patients with CLTI remaining, who had CLTI till the end of follow-up and again 1 more patient got worse till the 8 months follow-up with recurrence of CLTI. At the end of follow-up there were 3 patients with CLTI and 8 patients (Table 3) with positive treatment effect (equal to 72.7%). If we evaluate the Rutherford classification we can see a slight difference in comparison with intravenous sub-group-starting with average Rutherford 4.36 we see an improvement to 2.91 at 4 months but there was no case of worsening or CLTI recurrence at 8 months (as it was in intravenous sub-group). Here we see the same situation as in claudication group with further improvement in Rutherford class up to 2.55. This means the overall improvement of- 1.81 Rutherford class (p=0.003) (Table 4).
| Rutherford improvement 4 month | Rutherford improvement 8 month | |
|---|---|---|
| Total | (-) 1.84 | (-) 1.79 |
| Intravenous | (-) 2.00 | (-) 1.78 |
| Oral | (-) 1.45 | (-) 1.81 |
If we compare Rutherford class between both sub-groups we can see an obvious difference at 4 months with Rutherford class improvement in intravenous subgroup-2.00 and in oral subgroup only-1.45, but statistically it was not rated significant (p=0.118). At 8 months this difference diminishes with intravenous sub-group worsening to-1.78 (compared with baseline) and there is a further improvement in oral subgroup to-1.81 (p=0.963).
Discussion
In accordance with literal sources and published clinical trials [15-19,24] we observed overall positive effect of Sulodexid medication in cases of patients indicated for conservative treatment of PAD, in our retrospective study even in cases of patients with CLTI.
We observed a significant improvement in PFWD in almost all patients with claudication pain. If we consider relative improvement from baseline value of PFWD there is more significant improvement in cases of intravenous start of the therapy (5 times more than baseline), but we have to consider other facts involved in this outcome. At first intravenous sub-group started with almost 3 times shorter PFWE (71 vs. 191 m) and thus even when there was almost the same improvement in PFWD for both sub-groups (291 vs. 293 m) it yields much higher ratio for intravenous sub-group. The same conclusion can be said about Rutherford classification comparing both sub-groups. In intravenous subgroup the improvement was-1.0 Rutherford class compared to-0.7 class in oral sub-group, but again, there was worse starting point for intravenous sub-group (2.93 vs. 2.40) and in this case we must admit that Rutherford classification has a rough scaling (200 m) and thus even a small change in PFWD can mean the same improvement as a much bigger change in another case. This is the reason why we consider absolute improvement in PFWD as the most objective parameter of evaluation Sulodexide therapy effect on patients with claudication pain. The same improvement of absolute value of PFWD in both subgroups can be explained by a relatively good response of collateral arterial branches to the antithrombotic and profibrinolytic effects of Sulodexide as well as endothelium protective effect and the rheological effect. Particularly in cases of patients with claudication in which the collateral branches can reach its full blood flow potential quickly regardless how the therapy starts.
Another positive observation was the fact that PFWD improved more with longer therapy period (both sub-groups counted together at the baseline had a PFWD 144 m which improved to 376 at 4 months and to 430 m at 8 months). Our theory behind this observation is a good capacity of collateral arterial branches and effects of Sulodexide on vessels (antithrombotic, profibrinolytic, endothelium protective) in cases of claudication patients with atherosclerotic damage located mostly in the main arterial trunks. In such cases Sulodexide (together with physical training) could positively influence the formation of collateral blood flow and thus improve a perfusion of lower limb even if the main arterial trunks are stenotic or occluded [25]. Nevertheless, we can’t take this effect as granted, because there were 4 cases of worsening of PAD status during the follow-up and one of them even to state of CLTI.
In every case of patient with CLTI and with no possibility of revascularization we offer intravenous vasodilatation therapy (Agapurin, Procain) or in last three years mostly Sulodexide therapy in effort to improve a perfusion of ischemic limb. We observed significant improvement in CLTI group (both in intravenous and oral subgroup) relieving 71.0% of the patients of CLTI symptoms (rest pain of ischemic wound) during 8 months of therapy. Because there are very limited literal sources dealing with Sulodexid therapy in cases of CLTI [26] we were not able to compare our findings with any other source. Despite of the fact that this is a retrospective study it is unique in this way.
To evaluate an effect of Sulodexide therapy in CLTI cases we chose to compare a CLTI status (“yes” or “no”) and the change in Rutherford class. Comparing PFWD in these cases would be ineffective and confusing because most of the patients are limited in walking by the presence of ischemic wound or the pain which is present even before they start walking.
A majority of the CLTI patients started the therapy with intravenous application because their condition required more intensive approach, even in conservative point of view. Even if we observed relatively bigger effect of the therapy at 4 months for intravenous sub-group (-2.0 vs.-1.45 Rutherford class) it was not statistically significant with p=0.118. And this difference diminished at 8 months when the improvement from baseline was-1.78 for intravenous and-1.81 class for oral sub-group. These interesting findings with bigger effect of intravenous therapy during the shorter period and worsening in longer period compared too slowly but steadily improving effect of oral therapy can be explained by two possible theories. Intravenous therapy was used even in cases of subacute critical limb ischemia (subacute rest pain) with no technically possible revascularization. In these situations, the Sulodexide therapy can lead to relief from the rest pain and thus fast improvement in Rutherford class. On the other hand, oral therapy was used in cases of patients with a long time nonhealing but stabilized ischemic wound as an attempt to improve the healing in outpatient setting, what is much longer process than rest pain elimination in subacute state. This can be one of the reasons why the oral therapy improved over longer time as more wounds healed. Another reason for this difference could be much sever atherosclerotic arterial damage in CLTI patients, which progress more quickly than in claudication group and it overwhelms effect of the Sulodexid therapy in longer follow-up. Although this theory doesn’t explain why there was still improvement in oral sub-group, but it can explain worsening of CLTI and Rutherford class in intravenous sub-group between 4 and 8 months.
There is an important fact influencing interpretation of improvement in Rutherford class in CLTI cases-vast majority of patients with ischemic wound were diabetics (87.5%). Diabetic neuropathy is surely one of the risk factors for wound creation, but it can also very negatively influence ranking a patient by Rutherford classification. If we have a patient with ischemic wound, he is rated Rutherford 5, but when the wound is healed patient loses all subjective symptoms (he feels no rest or walking pain) because of diabetic neuropathy. According to clinical symptoms this patient can be even rated Rutherford 0, what doesn’t reflect the severe atherosclerotic damage in his or her lower limb arteries. This fact can also distort evaluation of Sulodexide effect by Rutherford classification. Because of these circumstances we consider evaluating Sulodexide effect by a portion of patients relieved of CLTI to be more appropriate and accurate than improvement in Rutherford class.
Conclusion
During our retrospective study we verified overall positive effect of Sulodexid therapy for PAD patients, even in the cases of CLTI patients. Positive effect on improving PFWD is well known from previous trials and in approval with these trials we observed a significant improvement in PFWD both after intravenous and oral medication. What is even more important for clinical praxis is the effect of Sulodexid on CLTI. Sulodexide proved to be a potent option for conservative therapy in difficult cases of CLTI patients with no revascularization options. In our study most of the patients suffering from CLTI at the start of Sulodexide therapy were relieved of the rest pain or ischemic wounds. Sulodexid seems to be an effective auxiliary treatment in CLTI cases with rational cost/ benefit ratio. In cases of claudication pain it could be even used as standalone therapy (together with antiagregation medication and physical training) improving patient quality of life and postponing eventual intervention.
Conflict of Interests
The Authors declare that there is no conflict of interest.
Ethical Statement
There was no Ethic Committee approval required for the retrospective study of patient documentation. All patients give a written consent with use of their clinical data for research and educational purposes at the beginning of their treatment.
REFERENCES
- Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329-1340.
- Ruiz-Canela M, Martínez-gonzález ma. lifestyle and dietary risk factors for peripheral artery disease. Circ J 2014;78(3):553-559.
- Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509-1526.
- Hussein AA, Uno K, Wolski K, Kapadia S, Schoenhagen P, Tuzcu M, et al. Peripheral arterial disease and progression of coronary atherosclerosis. Circulation. 2010;122(21):A13431.
- Dumville JC, Lee AJ, Smith FB, Fowkes FGR. The health-related quality of life of people with peripheral arterial disease in the community: The Edinburgh Artery Study. Br J Gen Pract. 2004;54(508):826-831.
- Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. Jama-J Am Med Assoc. 2001;286(11):1317-1324.
- Kinlay S. Management of critical limb ischemia. Circ Cardiovasc Interv. 2016;9(2):e001946.
- Hillestad L. The Peripheral blood flow in intermittent claudication .4. significance of claudication distance. Acta Med Scand. 1963;173(4):467-478.
- Falluji N, Mukherjee D. Critical and acute limb ischemia: An overview. Angiology. 2014;65(2):137-146.
- Aboyans V, Ricco J-B, Bartelink M-LEL, Bjorck M, Brodmann M, Cohnert T, et al. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39(9):763-816.
- Aboyans V, Bjorck M, Brodmann M, Collet J-P, Czerny M, De Carlo M, et al. Questions and answers on diagnosis and management of patients with Peripheral Arterial Diseases: A companion document of the 2017 ESC guidelines for the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39(9):E35-41.
- Stegman BM, Shishehbor MH. Optimal revascularization for critical limb ischemia: One approach doesn’t always fit all. J Endovasc Ther. 2015;22(4):482-484.
- Novakovic M, Jug B, Lenasi H. Clinical impact of exercise in patients with peripheral arterial disease. Vascular. 2017;25(4):412-422.
- Lindgarde F, Jelnes R, Bjorkman H, Adielsson G, Kjellstrom T, Palmquist I, et al. Conservative Drug-Treatment in Patients with Moderately Severe Chronic Occlusive Peripheral Arterial-Disease. Circulation. 1989;80(6):1549-1556.
- Gaddi A, Galetti C, Illuminati B, Nascetti S. Meta-analysis of some results of clinical trials on sulodexide therapy in peripheral occlusive arterial disease. J Int Med Res. 1996;24(5):389-406.
- Shustov SB. Controlled clinical trial on the efficacy and safety of oral sulodexide in patients with peripheral occlusive arterial disease. Curr Med Res Opin. 1997;13(10):573-582.
- Lauver DA, Lucchesi BR. Sulodexide: A renewed interest in this glycosaminoglycan. Cardiovasc Drug Rev. 2006;24:214-226.
- Palmieri G, Nazzari M, Ambrosi G, Campiotti A, Palazzini E. Sulodexide in the Treatment of Peripheral Arterial-Disease. Clin Trials J. 1984;21(6):411-427.
- Coccheri S, Scondotto G, Agnelli G, Palazzini E, Zamboni V. Sulodexide in the treatment of intermittent claudication-Results of a randomized, double-blind, multicentre, placebo-controlled study. Eur Heart J. 2002;23(13):1057-1065.
- Harenberg J. Review of pharmacodynamics, pharmacokinetics, and therapeutic properties of sulodexide. Med Res Rev. 1998;18(1):1-20.
- Mattana P, Mannello F, Ferrari P, Agus G. Vascular pathologies and inflammation: The anti-inflammatory properties of sulodexide. Ital J Vasc Endovasc Surg. 2012;19:1-7.
- Tetteroo E, Engelen AD, Mali WP. Feasibility of the SVS/ISCVS classification ('Rutherford categories’) for chronic lower limb ischemia and percutaneous treatment results. Radiology. 1999;213P:433-433.
- Mills JL, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk stratification based on Wound, Ischemia, and foot Infection (WIfI). J Vasc Surg. 2014;59(1):220-34.e1
- Lasierra-Cirujeda J, Coronel P, Aza M, Gimeno M. Use of sulodexide in patients with peripheral vascular disease. J Blood Med. 2010;1:105-114.
- Murrant CL. Structural and functional limitations of the collateral circulation in peripheral artery disease. J Physiol-Lond. 2008;586(24):5845.
- Piaggesi A, Abbruzzese L, Mattaliano C, Bargellini I, Cicorelli A, Iacopi E, et al. Sulodexide as adjunctive therapy in diabetic foot patients with critical limb ischemia treated with percutaneous transluminal angioplasty. Int J Low Extrem Wounds. 2014;13(2):103-109.
Citation: Kubicek L, Dvorak M, Staffa R (2021) Validating Effect of Sulodexide in Treatment of Claudication Pain and Chronic Limb-Threatening Ischemia. J Vasc Med Surg. 9:409.
Copyright: ©2021 Kubicek L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.