Indexed In
- Open J Gate
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 7, Issue 1
Tri-Carboxylic Acid Red Onion (Allium cepa) Skin Extract Resin for the Removal of Chromium (VI) Ion from Aqueous Solution
Ibezim-Ezeani Millicent Uzoamaka*, Onyeogulu Chibuike and Akaranta OnyewuchiReceived: 22-Dec-2018 Published: 12-Mar-2019
Abstract
This study investigates the removal of chromium (VI) ions from aqueous solution using resin synthesized by modifying red onion skin extract with a tri-carboxylic acid (citric acid). The performance of citric acid red onion skin extract resin (CRER) in the removal process was examined based on the effect of temperature (302 to 343 K), agitation time (10 to 60 min) and initial ion concentration (2 to 100 mg/L). Data analysis from agitation time experiments showed that equilibrium adsorption was attained within 30 min. Maximum percentage adsorption was observed at 313 K, while the percentage ion adsorbed increased with increase in initial concentrations of Cr (VI) ions. The experimental data were subjected to Langmuir, Freundlich and Temkin isotherm models evaluation; and the deductions from the correlation coefficient (R2) of the plots showed that Freundlich isotherm model represented the best fitting for the batch experiment. Thermodynamic parameters were determined and the results indicated that the sorption process was spontaneous with ΔG values as -9.433, -8.811, -7.745, -5.400 and -3.395 kJ/mol for 302, 313, 323, 333 and 343 K respectively. Adsorption with CRER was exothermic with respect to its negative ΔH (-55.610 KJ/ mol) value, while decrease in disorderliness of the adsorption system was reflected by the negative ΔS (-150.816 J/K/ mol) value. Pseudo-second order kinetic process was resolved for the reaction rate investigations, with the values of activation energy and pre-exponential factor as -118.167 kJ/mol and 5.749 × 10-17 g/mol/min respectively. Dubinin- Radushkevich isotherm model was used to evaluate the sorption mechanism, and it was observed that physisorption is the dominant mechanism for the reaction with the E values ranging from 6.51 to 6.95 kJ/mol at the different experimental temperatures.
Keywords
Citric acid; Red onion skin; Chromium (VI) ion; Pseudo-second order; Dubinin-Radushkevich isotherm
Introduction
The earth surface is 70% covered by water of which about 97% of it is salty water and only 3% of the world water is fresh. This fresh water is 70% frozen, and only less than 1% of the water is available for drinking and domestic purposes [1]. Urbanization and industrialization further possess waste water disposal problem, as most of the industrially generated wastes contain very high concentration of heavy metal ions that end up in water bodies. The occurrence of these heavy metals has the ability of enhancing different soil characteristics and the migration of toxic ions through dissolution of metals [2].
Studies have shown that an estimate of 1.2 billion humans drink contaminated water which makes it a channel for the prevalence of water related sickness in the society, which eventually leads to the death of approximately 5-10 million people around the world, most of whom are children [3]. In developing countries, statistics have shown that over 70% of generated industrial waste is dumped untreated into water bodies, thus polluting the useable water supply. This industrial waste only adds to the increase of other toxic pollutants that contaminate and reduce the water beneficial properties [4].
Pollution of water bodies by heavy metals poses serious threat in aquatic ecosystem because majority of these metals are highly poisonous, even at very small concentrations. Nonbiodegradability and bioaccumulation of metal in living
organism have made them to become concentrated throughout the food web, thereby increasing the tendency of heavy metal ions adsorption into human body, resulting in various effects on human health worldwide [5,6]. It is of utmost importance that the level of these metal concentrations in portable waters, effluent waters, and waters used for agricultural and recreational purposes be reduced to the range recommend and recognize by International Health Authorities such as the World Health Organization (WHO).
Heavy metals such as cadmium, chromium, nickel, lead and zinc have been narrated to be introduced into immediate surroundings through media such as air, food and soil and water [7]. This heavy metal have been studied and reported to damage the ecosystem, affect human health due to its hazardous nature, especially when their concentration is more than the accepted limit [8-10]. There major sources include waste-water discharged from health centers, different production industries including Cd-Ni battery, metal plating and alloy producing [11]. Research has shown serious environmental concern due to the presence and effect of these metals in waste stream and ground water because these metals are toxic to various life forms; however, removing them and controlling their levels in waste waters is very crucial [12].
Chromium is the first element in group six of the periodic table, with an atomic number of 24. Its characteristics include luster, hard, brittle and steely-grey in color [13]. It is a chemical element that takes a high polish resistance furnishing and has a high melting value. Chromium, being a transition metal and belonging to period four in the periodic table has a standard atomic weight of 52 g/mol, melting point 2180 K, boiling point 2944 K and density 7.19 g/cm3. It has different oxidation states mainly +6, +3 and +2, as well as different isotopes (50Cr, 51Cr, 52Cr, 53Cr and 54Cr) [14]. Chromium metal possess a high value as a result of its hardness and high corrosion resistance, thus having an electronic configuration of [Ar] 3d5 451.
Chromium metal ion in its +3 oxidation state has not been considered toxic. Cr (III) ion is a good nutrient in small amounts in humans and plays a role in metabolism of insulin and sugar. However, Cr (VI) has been considered toxic and cancer causing. Trivalent chromium is a dominant species compare to hexavalent chromium. The relation between Cr (III) and Cr (VI) solely depends on oxidative properties and pH with regard to its location. Chromium +3 oxidation state has higher stability, +3 and +6 are the most common among chromium compounds [15]. Inhaled hexavalent chromium is a known human carcinogen. People exposed to hexavalent chromium are at higher risk of developing lung cancer, asthma, or damage to the nasal epithelia and skin. Cancer in the oral cavity and small intestine has also been caused by drinking of contaminated chromium (VI) water. Also irritation or ulcers in the stomach and liver, and toxicity of the liver are all caused by accumulation of contaminate chromium (VI) water [16]. In the United States, the Occupational Safety and Health Administration permissible exposure limit for airborne exposure of hexavalent chromium is 5 μg/m3 (0.005 mg/m3) [17].
Several water treatment methods are available, which include: adsorption, ion exchange, chemical precipitation, membrane process, flotation, chemical coagulation and electrochemical methods [5]. These methods have series of limitations when considering the degradability of sorbent, ability to bind and concentrate metal ions from aqueous media [18]. Thus, the application of adsorption technique using adsorbents of biodegradable origin offer outstanding number of advantages such as ease of operation, economical in terms of cost of application, green chemistry oriented, compactible with ecosystem and high degree of the treated effluent especially for well-designed sorption process [19,20]. Nevertheless, since the process of the metal ion removal involves interaction of the metal ions concentrate and the active groups within the adsorbent; simple chemical processes are usually employed in the retrieval of metal ions from the used adsorbent [21].
The characteristic behavior of carboxylic acid compounds and their derivatives have long history of usefulness in the production of additives, preservatives, carbonated beverages, fruit juice, dairy products, drugs, fungicides, plastics lubricants, paints, processed food, resins, varnishes, adhesives, photographic films, lubricating oils, rubbers, perfumes, paraffin candles, etc. Citric acid was used in this research so as to maximize its hydroxyl group property. In this case study therefore, the metal removal capacity of citric (tri-carboxylic) acid modified red onion skin extract resin (CRER) in relation to the influence of pH, concentration, agitation time and temperature on the process was explored for the remediation of Cr (VI) ion contaminated water.
Materials and Methods
Sample collection and preparation
The purchased onion skin from mile 1 market in Port Harcourt, Rivers State, Nigeria was preserved in a clean plastic bag, and transported to the laboratory for the research. The onion skin was washed with distilled deionized water and aired for three days at 302 K to dry. Milling of the dried red onion skin was performed with an electric grinding machine, sieved to particle size of 150 μm, and preserved in corked bottle prior to extraction process.
Extraction procedure
Extraction of pulverized red onion skin (3450 g) was carried out using soxhlet extractor and acetone as the extracting solvent. The desired extract was recovered from the extract-solvent mixture by evaporation.
Preparation of citric acid red onion skin extract resin (CRER)
Red onion skin extracts (15 g) was dispersed in 100 ml of deionized water at 302 K for 30 min on a magnetic hot plate with constant stirring. Citric acid (4.77 g) was added to the stirred mixture and the temperature was increased to 323 K. Iodine of 0.75 g was added to the mixture, with the temperature of the water bath maintained at 323 K for 4 hours. The reaction mixture was removed from the heating source and allows cooling to 302 K. A 30 ml saturation solution of Na2S2O4 was added to the mixture and the brownish color of the iodine thrice with 250 ml distilled deionized water. The resultant residue was dried for 4 hours at 333 K in an oven, and stored in an airtight container properly labeled at 302 K [22].
Preparation of standard solutions
Stock solution (1000 mg/L) was prepared in 1 L volumetric flask by dissolving 5.73 g of Na2Cr2O7.2H2O in distilled deionized water. Standard solutions of 5-100 mg/L Cr (VI) ion solutions were prepared by dilution of the stock.
Adsorption capacity studies
Batch equilibrium experiments were carried out to determine the adsorption capacity with focus on the effect of temperature, agitation time and concentration. The experiment involves shaking 0.2 g of CRER with 20 ml of the Cr (VI) ion solution in a mechanical shaker with corked plastic bottle at 302 K. The mixture was equilibrated for an hour and filtered. Analysis of the filtrate was done with Atomic Absorption Spectrophotometer (Perkin Elmer, 700 series) to ascertain the amount of metal ion adsorbed by CRER. The above experiment was repeated at different concentrations (2 to 100 mg/L), agitation time (10 to 60 min) and temperature (29 to 70°C).
Analysis of metal ion adsorption capacity
The subtraction of the value of equilibrium concentration from that of initial metal ion gave the amount of metal ion adsorbed, while the percentage Cr (VI) ion adsorbed by CRER was calculated using equation (1).
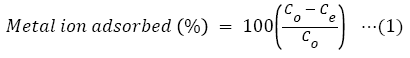
The adsorption capacity (qe) of CRER was deduced by the mass balance from initial to equilibrium composition using the relationship in equation (2) and the values plotted against those of initial metal ion concentration [23-25].
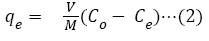
Where Co and Ce are the initial and final equilibrium metal ion concentration in solution (mg/L) respectively; V is the volume of the initial metal ion solution (L) and m is mass of CRER used (g).
Determination of point of zero charge with CRER
The point of zero charge of CRER was evaluated using the procedure [26]. 0.1 g sample of CRER was placed in several conical flasks containing 50 cm3 of 0.01 M NaCl. The pH value of the suspension was adjusted to 2, 4, 6, 8, 10, 12 by adding drops of 0.1 M HCl or 0.01 M NaOH solutions. The tightly corked flask was agitated intermittently in a mechanical shaker at 302 K for an hour to attain equilibrium. The suspension was filtered and the final pH of the filtrate determined. The ΔpH is then plotted against the initial pH, and the point of zero charge extrapolated from the plot.
Results and Discussion
Point of zero charge
The point of zero charge (PZC) is the pH at which a resin dispersed in an electrolyte displays neutrality or zero charge. However, for a resin to be able to take up positive metal ions (cations), it needs to have pH value above the PZC value; thus, making the medium negatively charged, thereby adsorbing or exchanging positive metal ions.
From the experiment, CRER exhibited PZC value of 4.7, showing that at pH greater than 4.7, CRER resin can successfully adsorbed Cr (VI) metal ions in an aqueous medium (Figure 1).
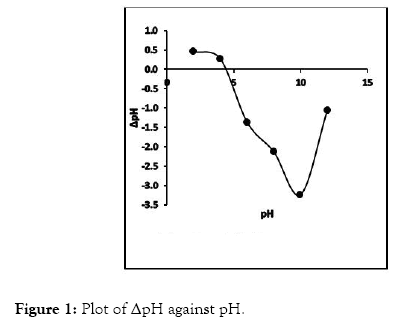
Figure 1: Plot of ΔpH against pH.
Effect of temperature
Temperature effects on adsorption of metal ion onto CRER were studied at temperatures of 29, 40, 50, 60 and 70°C. The experimental result for CRER was not linear with regards to temperature increase. It was observed that temperature increase from 29 to 40°C increased the percentage ion adsorbed from 90.70 to 98.90% (Figure 2).
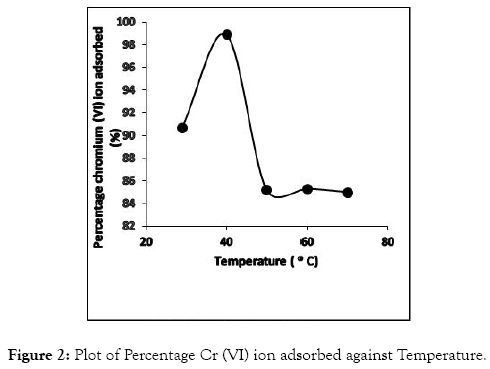
Figure 2: Plot of Percentage Cr (VI) ion adsorbed against Temperature.
However, with further increase in temperature, the percentage ion adsorbed reduced gradually from 98.90 to 85.22% for 40 to 50°C; followed by 85.30 to 85.00% between 50 and 70°C. This suggests weak interaction of metal ions with the CRER active points and also between the adjacent molecules of the adsorbing phase at temperature above 40°C. The CRER adsorbents experienced some form of deformation of its pore size due to increase in temperature, thus leading to alteration of the original structural arrangement of the resin matrix [27].
Effect of agitation time
The degree of metal ion adsorption is related to the efficiency of active sites to adsorb metal ions, as well as the contact time at the solid-liquid interface [28]. The result for the time dependent experiment for the adsorption of Cr (VI) ions at 29°C are shown in Figure 3.
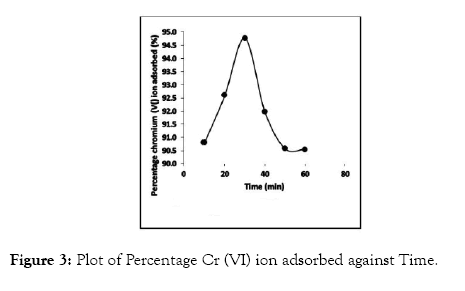
Figure 3: Plot of Percentage Cr (VI) ion adsorbed against Time.
Cr (VI) ion adsorption rate was high at the initial stage of the analysis such that more than 90% of total ion adsorption occurred within 30 min, after which adsorption decreased and followed by no significant metal ion uptake between 50 and 60 min. This could be due to the large surface area of CRER adsorbing site at the beginning resulting to ion adsorption at the active surface. In addition, metal ions tend to pass via the deeper surface of the adsorbent pores for ion adsorption and encounter much large resistance which slows down the adsorption process during the later phase of adsorption [29]. The adsorption reduced to 91.97, 90.58 and 90.55% at time 40, 50 and 60 min respectively after equilibrium was attained at 30 min (94.77%). The experimental result showed that agitation time of 30 min was enough to achieve maximum adsorption, since there was no drastic change observed in percentage adsorption after 30 min.
Effect of initial metal ion concentration
Adsorption of metal ion is sufficiently influenced by the initial metal ion concentration in aqueous solution. In this study, initial metal ion concentration was varied from 5-100 mg/L and its effect on the adsorption of Cr (VI) ions is presented in Figure 4. In order to obtain the sorption capacity of CRER, Cr (VI) metal ion data was plotted as the percentage concentration of the metal ion adsorbed with the adsorbent against the initial metal ion concentration (Co). The graph representing the results is presented in Figure 4.
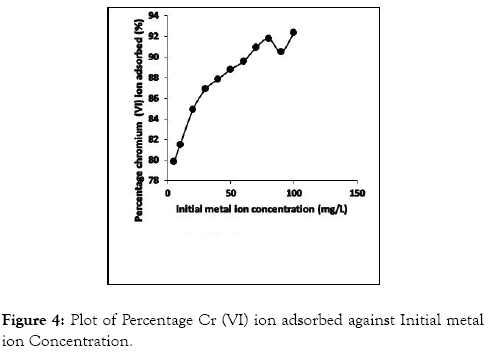
Figure 4: Plot of Percentage Cr (VI) ion adsorbed against Initial metal ion Concentration.
It is observed from Figure 4 that the degree of adsorption is a function of initial metal ion concentration. The percentage ion adsorbed for CRER increased with increase in initial concentration of Cr (VI) ions. This may result from an increase in metal ions for the specific amount of adsorbing material and due to saturation of available adsorption site on the adsorbing material.
However, increase in Cr (VI) ion adsorption as the initial metal ion concentration increase could be because, at lower initial concentration the few Cr (VI) metal ions present in the solution interact with the available active points, thus enhancing the ion adsorption. Furthermore, as initial metal ion concentration increases, and with functional active points remaining constant; the percentage ion adsorbed increased drastically due to the presence of much available metal ions in solution competing for the fixed number of active sites on the CRER until ion adsorption maximum is attained; thus, points out to the saturation of the active sites with some metal ions unabsorbed from solution [30].
Sorption thermodynamic studies
The fundamental parameters necessary for the design of any system of heavy metal removal involves the equilibrium data evaluated from studies on sorption dynamics. Sorption isotherms describe how the sorbate interacts with sorbents [31]. Equilibrium is established at the optimum sorption capacity of sorbents (CRER) and sorption isotherm helps in evaluating the sorption capacity of sorbents from the batch experiment. The isotherm which best describes sorption process was investigated by applying the equilibrium results evaluated at temperatures 302, 313, 323, 333, and 343 K to the linearized forms of Langmuir, Freundlich and Temkin models [32-37]. These models are presented in equations (3), (4) and (5) respectively.
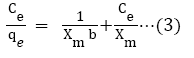
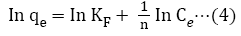
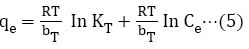
The constants b, KT and KF are for Langmuir, Temkin and Freundlich isotherm respectively, Xm is the monolayer adsorption capacity, 1/n is the activity strength of adsorption in the sorption process having values between zero and one, R is the gas constant and T is temperature, bT is related to the adsorption rate, Ce is the equilibrium concentration of the solute in the solution (mol/L), and qe is the amount of solute adsorbed (mol/g). The plots of the adsorption isotherms are display in Figures 5-7.
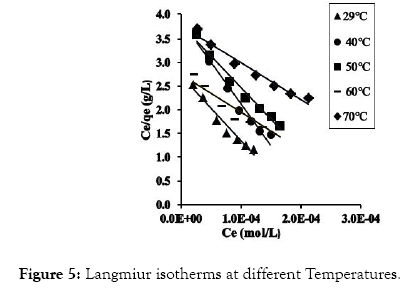
Figure 5: Langmiur isotherms at different Temperatures.
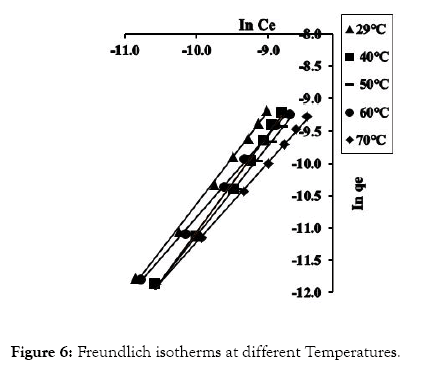
Figure 6: Freundlich isotherms at different Temperatures.
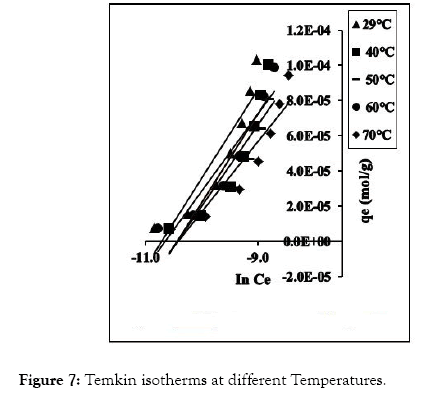
Figure 7: Temkin isotherms at different Temperatures.
The linear equations for the three isotherm models and their respective correlation coefficient (R2) values are presented in Tables 1 and 2. Freundlich isotherm model gave the highest R2 values and best explained the adsorption process.
| Temp (K) | Langmuir | Freundlich | Temkin |
|---|---|---|---|
| 302 | y=-13666x +2.6962 | y=1.4389x+3.7570 | y=5E-05x +0.0005 |
| 313 | y=-14342x +3.4669 | y=1.4349x +3.3857 | y=5E-05x +0.0005 |
| 323 | y=-13307x +3.7858 | y=1.3997x +2.8844 | y=5E-05x +0.0005 |
| 333 | y=-78850x +2.7300 | y=1.2771x +1.9507 | y=4E-05x +0.0005 |
| 343 | y=-77620x +3.7706 | y=1.2404x +1.1908 | y=4E-05x +0.0005 |
Table 1: Linear Equations of the Isotherm Model.
| Temp (K) | Langmuir | Freundlich | Temkin |
|---|---|---|---|
| 302 | 0.9572 | 0.9967 | 0.8641 |
| 313 | 0.828 | 0.9877 | 0.8567 |
| 323 | 0.9796 | 0.9963 | 0.852 |
| 333 | 0.8513 | 0.9983 | 0.8993 |
| 343 | 0.9705 | 0.9988 | 0.8729 |
Table 2: Comparison of Coefficient of Determination (R2) for the Isotherms.
However, it signifies that CRER possess a heterogenic surface. Again, Freundlich isotherm model was explored in evaluating n, 1/n, KF and ΔG at the different temperatures and their values presented in Table 3.
| Freundlich Constant | ||||
|---|---|---|---|---|
| Temp (K) | n | 1/n | KF (mol/g) | ∆G (KJ/mol) |
| 302 | 0.695 | 1.4389 | 42.82 | -9.433 |
| 313 | 0.6949 | 1.4349 | 29.539 | -8.811 |
| 323 | 0.7144 | 1.3997 | 17.893 | -7.745 |
| 333 | 0.783 | 1.2771 | 7.034 | -5.4 |
| 343 | 0.8062 | 1.2404 | 3.29 | -3.395 |
Table 3: Freundlich constants and ΔG Values at different temperatures.
From Table 3, it was observed that the values of 1/n with respect to all temperature studied are greater than unity, which indicates that cooperative sorption process played a vital part in the removal of Cr (VI) ions using CRER. Also, the n results are less than one referring to the fact that bond energies increase with the surface density. Similar trend in the removal of Cd (II) and Pb (II) ions by using succinic acid red onion skin extract resin [38].
The plot of In KF against 1/T is linear (Figure 8). From the graph, ΔH and ΔS were evaluated from the slope and intercept using equations (6) [39]. ΔG was computed with equation (7).
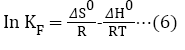
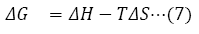
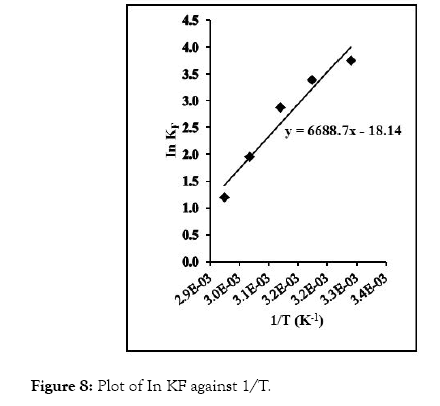
Figure 8: Plot of In KF against 1/T.
The ΔH value evaluated from the slope of the graph in Figure 8 is -55.610 kJ/mol, while ΔS value from the intercept is -150.816 J/K/mol. The ΔG value as calculated at different temperatures is shown in Table 3. The negative ΔH values imply that exothermic sorption process occurred, while the negative ΔS value indicates a reduction in randomness at resin-solution interface. Also, ΔG values were negative which indicate the feasibility and spontaneity of the adsorption process at the different temperature investigated. Negative ΔH and ΔS were observed in the removal of Cu (II) ions from aqueous solution by the use of onion and garlic skin extracts; while negative ΔG and positive ΔS were observed in the removal of Mn (II), Fe (II), Pb (II) ions from aqueous medium using carboxylatedepichlorohydrin red onion skin extract resins [40,41].
Adsorption kinetic studies
Adsorption kinetics is a necessary parameter for evaluating the adsorption pathway and efficiency in the removal of toxic metal ions from aqueous medium. In this study, pseudo-first order, pseudo-second order, Elovich and intra particle diffusion model, were used to evaluate and predict the sorption behavior of the batch analysis. An equation (8) to (11) represents the linear equations of the models [42-46].
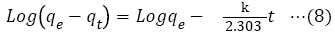
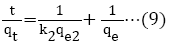
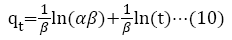
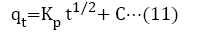
qe=amount of the chromium (VI) ions adsorbed at equilibrium;
qt=amount of the chromium (VI) ions adsorbed (mol/g) at time (t);
k1=pseudo-first order rate constant adsorption (1/min);
k2=pseudo-second order rate constant adsorption (g/mol/min);
α=initial adsorption rate (mol/g/min);
β=desorption constant (g/mol);
kp=intraparticle diffusion rate constant (mol/g/min-0.5).
The resolved values from the straight line graphs for adsorption kinetic studies are presented in Figures 9-12. The equation of straight line for the four models studied and their corresponding correlation coefficient (R2) are shown in Tables 4 and 5.
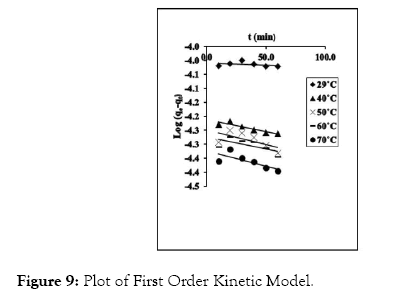
Figure 9: Plot of First Order Kinetic Model.
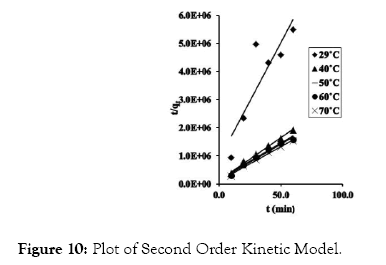
Figure 10: Plot of Second Order Kinetic Model.
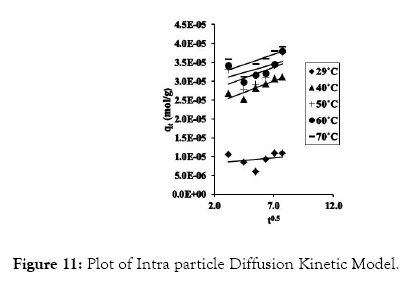
Figure 11: Plot of Intra particle Diffusion Kinetic Model.
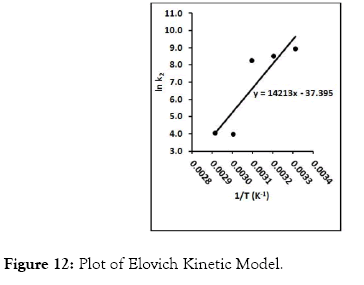
Figure 12: Plot of Elovich Kinetic Model.
| Temp (K) | Pseudo-first order | Pseudo-second order | Intraparticle diffusion | Elovich |
|---|---|---|---|---|
| 302 | Y y=-0.0020x-4.0092 | y=82629x+886999 | y=3E-07x+8E-06 | y=4E-07x+8E-06 |
| 313 | Y y=-0.0010x-4.2037 | y=29374x+174968 | y=1E-06x+2E-05 | y=3E-06x+2E-05 |
| 323 | y=-0.0011x-4.2480 | Y=25536x+168881 | y=1E-06x+3E-05 | y=2E-06x+2E-05 |
| 333 | y=-0.0009x-4.2735 | y=25830x+123983 | y=9E-07x+3E-05 | y=2E-06x+3E-05 |
| 343 | y=-0.0011x-4.3234 | y=24470x+102930 | y=1E-06x+3E-05 | y=2E-06x+3E-05 |
Table 4: Linear Equations for the Kinetic Model.
| Temp (K) | Pseudo-first order | Pseudo-second Order | Intraparticle diffusion | Elovich |
|---|---|---|---|---|
| 302 | 0.1118 | 0.7732 | 0.0552 | 0.6158 |
| 313 | 0.656 | 0.9841 | 0.5876 | 0.506 |
| 323 | 0.4373 | 0.9626 | 0.3109 | 0.1972 |
| 333 | 0.4054 | 0.976 | 0.2862 | 0.1786 |
| 343 | 0.5073 | 0.9905 | 0.4565 | 0.3422 |
Table 5: Coefficient of Determination (R2) Values for the Kinetic Model.
Pseudo-second order kinetic model gave the highest R2 values from the study, and thus gives a better explanation for the kinetic behavior of CRER in removing Cr (VI) ions from the metal solution. The pseudo-second order rate constant (k2) and qe were determine from the slope and intercept of the graph. In addition, the natural logarithm of k2 was plotted against 1/T at varied temperatures according to equations 12 (Figure 13) according to equations 12.
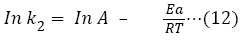
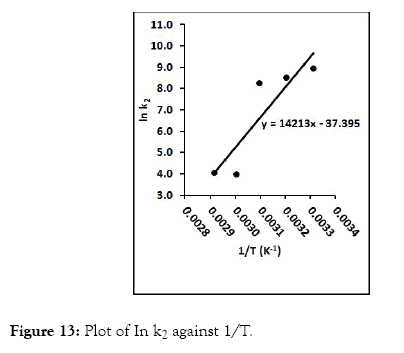
Figure 13: Plot of In k2 against 1/T.
The pre-exponential factor (A) and activation energy (Ea) was determined from slope and intercept of the plot as 5.749 × 10-17 g/mol/min and -118.167 KJ/mol respectively. The negative values of the activation energy indicate that exothermic sorption process took place.
Adsorption mechanism studies
Dubinin-Radushkevich (DR) isotherm model was used to study the pathway through which the reaction followed for removal of Cr (VI) ions from aqueous medium at different temperatures [47]. The linear form of DR isotherm model is displayed in equation 13:
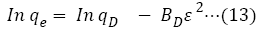
qD=maximum ion adsorption capacity (mol/g)
BD =DR model constant (KJ/mol)
ɛ=Polanyi potential which is equivalent to RT In(1+1/Ce), BD is related to the mean free energy E, of sorption per mole of metal ion through equation 14:
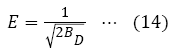
Plot of In qe versus ɛ2 gave linear graph (Figure 14).
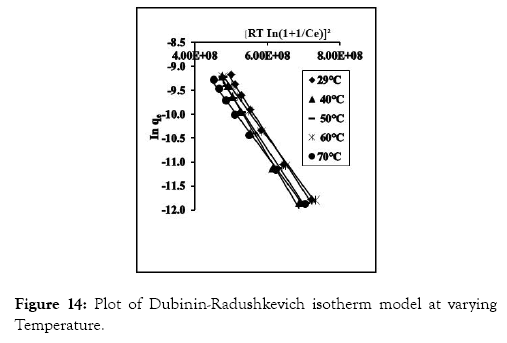
Figure 14: Plot of Dubinin-Radushkevich isotherm model at varying Temperature.
The values of BD and qD were evaluated from the slope and intercept of the plots. E values were deduced by inputting values of BD into equation (16). These values are displayed in Table 6. The E value gives information on the sorption pathway involved. If the E value is less than 8 KJ/mol, it implies physisorption is the dominant pathway, values ranging from 8 to 16 KJ/mol, implies sorption pathway involves ion exchange and above 16 KJ/mol, chemical adsorption is the dominant sorption pathway [48]. From the values in Table 6, the observed E values for CRER resin are below 8 KJ/mol, implying that physisorption is the main sorption pathway.
| Temp (K) | Linear equation | BD (mol2/J2) | qD(mol/g) | E (KJ/mol) | R2 |
|---|---|---|---|---|---|
| 302 | y=-1.179E-08x -3.3623 | 1.18E-08 | 0.0347 | 6.51 | 0.9933 |
| 313 | y=-1.150E-08x-3.5496 | -1.2E-08 | 0.0287 | 6.59 | 0.9911 |
| 323 | y=-1.151E-08x-3.8312 | -1.2E-08 | 0.0217 | 6.59 | 0.9925 |
| 333 | y=-1.089E-08x-4.2411 | -1.1E-08 | 0.0139 | 6.78 | 0.9974 |
| 343 | y=-1.034E-08x-4.6714 | -1E-08 | 0.0094 | 6.95 | 0.9958 |
Table 6: Linear Equations, Dubinin-Radushkevich Constants and R2 Values.
Conclusion
This study displayed the use of red onion skin extract in synthesizing resin for the adsorption of Cr (VI) from aqueous medium. Chemical modification of red onion skin extract using citric acid enhanced the adsorption characteristics. Batch experimental results shows that factors such as agitation time, initial metal ion concentration and temperature affect the metal removal capacity of CRER. Comparing the isotherm models applied for this research, Fruendlich isotherm model showed best fitting for the experiment with respect to the R2 value. The adsorption process was observed to be feasible with negative ΔG, exothermic with respect to its negative ΔH value. However, the negative ΔS value indicates reduced disorderliness. Kinetically, the adsorption process indicated that pseudo-second order model best described the experimental results with relatively low energy of activation; while the adsorption pathway was deduced to be physisorption using DR isotherm. This study has shown that CRER exhibit good adsorption capacity which may warrant its application in the remediation of Cr (VI) ion contaminated waste water.
Acknowledgement
The authors are grateful to Dr. Ikodiya Orji of the Department of Pure and Industrial Chemistry, University of Port Harcourt for her contributions during the experimental stage.
REFERENCES
- Smith A. Global Dialogue on Nanotechnology and the poor: Opportunities and Risks Chemistry International Issue Meridian Institute. 2009.
- Selivonovskaya S, Latypova V. The use of Bioassay for evaluating toxicity of sewage sludge and sewage sludge-amended soil. J Soil Sediment. 2003;3:85-92.
- Ahaja S. Handbook of water purity and quality. Academic Press, New York, USA. 2009.
- Igwe J, Abia A. A bio separation process for the removal of heavy metals from waste water using biosorbent. J Afr Biotechnol.2006;5:1167-1179.
- Cha D, Song J, San D. Treatment Technologies. Water Environ Res. 1997;69: 676-689.
- Crini G. Recent development in polysaccharide base materials used as adsorbent in waste water treatment. Prog in Polym Sci.2005;30:38-70.
- Garba ZN, Ubam S, Babando AA, Galadima A. Quantitative assessment of heavy metals from selected tea brands marketed in Zaria Nigeria. J Phy Sci. 2005;26:43-51.
- Tuzen M, Sari A, Mendil D, Soylak M. Biosorptive removal of mecury (II) from aqueous solution using lichen (Xanthoparmelia conspersa) biomass: kinetic and equilibrium studies. J Hazard Mater. 2009;169:263-270.
- Alslaibi TM, Abustans J, Ahmad MA, Abu-Foul A. Cadmium removal from aqueous solution using microwaved olive stone activated carbon. J Environ Chem Eng. 2013;1:589-599.
- Verlicchi P, Galletti A, Petrovic M, Barcelo D. Hospital effluents as a source of emerging pollutants and overviews of micro pollutants and sustainable treatment options. J Hydrol. 2010;389:416-428.
- Khavidaki HD, Aghaie H. Adsorption of thallium (I) ions using eucalyptus leaves powder. Clean. 2013;41:673-679.
- Serencam H, Gundogdu A, Uygur Y, Kemer B, Bulut VN, Duran C, et al. Removal of cadmium from aqueous solution by Nordmann fir (Abies nordmanniana (Stev.) Spach. Subsp. nordmanniana) leaves. Bioresource technology. 2008;99:1992-2000.
- Meija J. Atomic weights of the elements (IUPAC Technical Report) Pure Appl Chem. 2016;88:265-291.
- Housecroft EC, Alan G. Inorganic chemistry. Pearson, New Jersey, United States. 2007.
- Norman G, Alan E. Chemistry of the Elements. Butterworth-Heinemann, Burlington, United States. 2002.
- Salnikow K, Zhitkorich A. Genetic and Epi-genetic Mechanism in Metal Carcinogensis and Carcinogenesis: Nickel, Arsenic and Chromium. Chemical Res Toxicol. 2008;21: 24-44.
- David B. Tracking hexavalent chromium in ground water. Sci. 2002;295:2024-2025.
- Liang S, Guo XN, Tian Q. Application of Orange peel Xanthate for the adsorption of Pb2+ from aqueous solutions. J Hazard Mater. 2009;170:425-429.
- Qucton KG, Bonifacio DJ, Cybelle M, Futalan F, Meng-Wei W. Removal of Cr (VI) and Zn (II) from aqueous solution using Kaolin- supported bacterial biofilm of Gram-negative E coli and Gram-positive staphylococcus epidermis. Sustainable Environ Res. 2018;28:2206-2213.
- Milan M, Lakdawala Y, Patel N. The effect of low cost material bagasse fly ash to the removal of COD contributing component of combined waste water of sugar industry. J Hazard Mater. 2012;4:852-857.
- Harmon G, Patrick R, Spittler T. Removal of heavy metal from polluted water using lignocellulosic agricultural waste products. Ind Biotechnol. 2007;4:366-374.
- Orji I, Ibezim-Ezeani U, Akaranta O. Utilization of red onion skin extract for remediation of lead (II) and cadmium (II) ions from aqueous solution. Int J Enhan Res Sci Technol Eng. 2016;5:283-291.
- Chidi O, Okike UN, Okoye PI. The use of organophilic bentonite in the removal of phenol from aqueous solution: Effect of preparation techniques. Mod Chem Appl. 2018;6:1-8.
- Chen Y, Dong A, Sun S, Gao J, Qian L. Reduction and removal of chromium (VI) in water by powered activated carbon. Mater. 2018;11:269.
- Zulkali MMD, Ahmed AL, Noralakma NH. Oryza Sativa L. Husk as Heavy metal Adsorbent Optimization with Head as model solution. Bioresour Technol. 2006;97: 21-25.
- Onyango M, Shim EW, Karthikeyan KG, Tshibalala MA. Adsorption mechanism of cadmium on Juniper Bark and wood. Bioresour Technol. 2004;98:588-594.
- Ibezim-Ezeani MU, Okoye AF, Akaranta O. Kinetic studies on the removal of some metal Ions from aqueous solution using modified orange mesocarp extract. Int J Water Resour Environ Eng. 2012;4:192-200.
- Kobyab E, Demirbasa M, Senturkb E, Ozkan T. Adsorption kinetics for the removal of chromium (VI) from aqueous solution on the activated carbons prepared from agricultural waste. S Afr J Sci. 2004;30:533-539.
- Srivastava N, Majumder C. Novel biofiltration methods for the treatment of heavy metals from industrial waste water. J Hazard Mater. 2008;151:1-8.
- Beatrice WW, Mwangi IW, Murungi J, Wanjau RN, Msagati TAM. Remediation of lead, cadmium and copper polluted water by onion skin (Allium cepa). Int J Agri Innov Res. 2016;4:2319-1473.
- Shokoohi R, Saghi M, Ghafari H, Had M. Biosorption of iron from aqueous solution by dried biomass of activated sludge. Iran J Environ Health Sci Eng. 2009;2:107-114.
- Freundlich F. Over the adsorption in solution. J Struct Chem. 1906;57:385-470.
- Langmuir T. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chemical Chem. 1918;40:1362-1403.
- Ibezim-Ezeani MU, Anusiem ACI. Thermodynamics of the adsorption of palmitate and laurate soaps onto some metal ore surfaces in aqueous media. Afr J Pure Appl Chem. 2011;6:49-54.
- Singh L, Ahmad A, Abdwahid Z. Equilibrium, thermodynamic and kinetic studies for removal of chromium from aqueous solution by grafted copolymer. Int J Civil Eng Geo-Environ. 2012;3:57-61.
- Garba ZN, Ugbaga NI, Abdullahi AK. Evaluation of optimum adsorption conditions for Ni (II) and Cd (II) removal from aqueous solution by modified plaintain peal (MPP). J Basic Appl Sci. 2016;5:170-179.
- Jebessa AG. Preparation and evaluation of adsorption effectiveness of peanut husk for the removal of fluoride ions from aqueous solution. Mod Chem Appl. 2018;6:1-5.
- Ibezim-Ezeani U, Milicent M, Orji I. Sorption studies on the remediation of cadmium (II) and Pb (II) ions contaminated water using succinic acid modified red onion skin extracts. Int J Innov Res Dev. 2017;6:117-127.
- Mohammed AA. Thermodynamics approach in the adsorption of heavy metals, thermodynamics interaction studies-solid, liquids and gases. Web of Science. 2011.
- Chowdhury A, Bhowal A, Siddhartha S, Datta D. Equilibrium, thermodynamic and kinetic studies for removal of copper (II) from aqueous solution by onion and garlic skin. Water. 2012;4:37-51.
- Ibezim-Ezeani U, Millicent M, Onyewuchi A. Thermodynamic feasibility of Mn(II), Fe (II), and Pb (II) ions exchange in aqueous medium by red onion (allium cepa) skin extract resin. Int J Chem Res. 2016;9:510-562.
- Lagergren S, Svenska BK. On the theory of so-called adsorption of dissolved substances. R Swed Acad Sci Doc. 1989;24:1-13.
- Ho YS, Mckay G. Sorption of dye from aqueous solution by peat. Chem Eng J. 1989;70:115-124.
- Weber WJ, Morris JC. Kinetic of adsorption on carbon from solution. J San Eng Dev 1963;89:375-793.
- Alemu A, Lemma B, Gabbiye N, Tadele M, Minyahl A. Removal of Cr (VI) from aqueous solution using vesicular basalt: A potential low cost waste water treatment system, Heliyon. 2018.
- Liu W, Yang L, Xu S, Chen Y, Liu B, Li Z, et al. Efficient removal of hexavalent chromium from water by an adsorption–reduction mechanism with sandwiched nanocomposites. RSC advances. 2018;8:15087-15093.
- Kul AR, Koyuncu H. Adsorption of Pb (II) ions from aqueous solution by native and activated bentonite: kinetic equilibrium and thermodynamic study. J Hazard Mater. 2010;179:332-339.
- Cestari AR, Vieira EFS, Vieria GS, Almedia LE. Aggregation and adsorption of reactive dye in the presence of amino surfactant and mesoporous aminopropylsilica. J Colloid Interface Sci. 2007;309:402-411.
Citation: Uzoamaka IEM, Chibuike O, Onyewuchi A (2019) Tri-Carboxylic Acid Red Onion (Allium cepa) Skin Extract Resin for the Removal of Chromium (VI) Ion from Aqueous Solution. Mod Chem Appl 7:266. doi: 10.35248/2329-6798.19.7.266.
Copyright: © 2019 Uzoamaka IM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


