PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 8
Three-Step Diagnostic Assessment Following Adverse Reaction due to X-ray Contrast Media: An 11-Year Study in 47 Patients
Joseph M. Baló-Banga1,2*, Martina Kádas1, Adrienn Kása-Kovács1 and Adrienne Vajda12Department of Dermatoallergy, Medical Center of Hungarian Defense Forces, Budapest, Hungary
Received: 28-Jul-2021 Published: 18-Aug-2021, DOI: 10.35248/2155-6121.21.12.261
Abstract
Background: The diagnostic evaluation of adverse reactions occurring after iodinated contrast media is still debated. Immediate, delayed-type immune reactions and non-immune adverse events have to be differentiated. Our aim was to assess a safe three-step routine procedure in a single-center teaching hospital 2010-2021 with a prospective “real- life” outlook.
Methods: Forty-seven patients after mild intermediate and severe adverse events were tested in three consecutive days. Skin prick tests with 2 to 5 undiluted media were followed at 24 hours by intradermal testing of 2 different concentrations each (10-2m and 10-3m). The results were red after both 20 and 70 minutes and at 24 hours. On day three one negative medium selected was 1/3 diluted and given intravenously under close observation.
Results: The suspected 32 immediate, 11 delayed hypersensitive and 4 non-immune mediated reactors showed 22.3% skin prick test and 62.7% intradermal test positivity, 11 patients were all negative. The 70 minutes reading of intradermal tests modified the results obtained by 20 minutes. In 44.1% of the cases more and more pronounced positivity was found. The highest response rate was achieved by 10-3m solution of contrast media alone or in combination with 10-2m concentration. The only higher (10-2m) revealed 21.2%, while the only late(24 hours) manifesting tests only 8.1% positivity. Intravenous provocation confirmed in 32/38(84.2%) cases the selected alternative. Six cases were mildly positive. After testing the “real-life” versatility was 85% within the next year.
Conclusion: The three-step workup shown in a single-center based study was proven useful and enabled repeated use of radio contrast media in problem patients.
Keywords
Iodinated; Allergy; Hypersensitivity; Immediate reactions; Non immediate reactions; SDRIFE; Drug provocation tests; Real life outcomes
Introduction
Iodinated X-ray contrast media are in everyday use in clinical medicine to visualize internal organs upon iodine’s property of high contrast density. The Adverse Reactions (ADR) to Radio Contrast Media (RCM) can be toxic or based on individual hypersensitivity. The former may occur as acute kidney injury 48-72 hours after introduction [1]. Rare toxic effects in the brain resulted in fatal outcome [2]. Hypersensitivity (HS) reactions are more frequent. An earlier European multicenter study came to the conclusion that at least 50% of them are caused by immunological mechanisms [3]. They can be divided into immediate (HIS within 1 hour) or non- immediate reactions with or without allergic skin manifestations A frequent missbelief regarding “allergy to molecular iodine” even among medical personnel has to be fought against [4]; Only organically bound iodine molecules can act as elicitors of HS reactions. Anaphylaxis, urticaria, Angioedema (ANO), rhinitis, dyspnea, and hypotension are common clinical phenotypes of IHS while Maculo Papular Exanthemas (MPE) are the major manifestation of non-immediate reactions [5,6]. In order to clarify the validity of uncertain data in patient’s history the question often arise how to avoid the Adverse Events (AE) in patients who needed repeated use of RCM because of their chronic illnesses. The aim of our studies was to perform a prospective two-step skin test procedure to predict the danger of various X-ray contrast media emerging from the personal history and to validate it by the reintroduction of negatively tested material. Together with the third diagnostic step –provocation- the results were then further compared to the “real life” events emerging upon their utilization.
Patients, Materials and Methods
Total 47 patients 19 men and 28 women were sent by the radiology, vascular surgery, cardiology, gynecology, oncology, surgery, and neurology, and dialysis center, urology departments of our academic teaching hospital between 2010-2021 with uncertain history of previous immediate or delayed Adverse Events (AE) to one or more RCM. The patients were supposed to undergo promptly either X-ray contrast enhanced Computed Tomography (CT), Angiography, Coronariography (CTA) or Digital Subtraction Angiography (DSA) for diagnostic purposes. For the 3-step testing 40 of 47 were hospitalized for 3 days. The remaining ones were tested on 3 consecutive days as out-patients (This was necessitated by COVID-19 pandemic regulations after March 11, 2020). The study was approved by the Bioethical Committee of our Teaching Hospital and registered at Clinical Trials. It was performed in accordance with the Declaration of Helsinki. All included patients gave their informed signed consent to participate. Out of the broad battery of RCM the following ionic monomeric solutions were involved with testing; Metrizoates (Gastrografin® Peritrast®). Non- ionic monomeric solutions; Iohexol, iopromid, ioversol, iobitridol, iomeprol, iopamidol and non-ionic dimeric iodixanol according to the patients’ history or scheduled as alternative substances. All solutions contained 300 to 400 mg/ml material and were stored at 4°C until use.
Step 1: Patients were Prick-tested (SPT) by 2 to 5 contrast media including the suspected culprit one using undiluted stock solutions against saline (negative control) and 10-3 molar histamine (positive control). The reading times were 20’, 70’ and 24 hrs. Positive tests were noted as erythema, urtica (growing >3 mm of initial diameter) or as >3 mm red papule at 24 hrs.
Step 2: After 24 hours Intra Dermal Tests (IDT) have been carried out with the same materials using two dilutions 10-2 and 10-3m against buffered saline (negative control) and 10-4 m histamine (positive control). Twenty to 40 μl test solutions were injected intradermal. The reading times were as above. Positive results were identified in particular if the reaction developed at 10-3m concentration and the initial bleb-size grew in time (from 0’ to 20’) by at least 3 mm [7].
Step 3: After the second reading at 24 hours one RCM was selected with no positivity at any previous readings and was diluted 1:3 at room temperature with saline. Ten milliliters were then administered IV within 2 minutes. Vital parameters were monitored continuously, and the patients were closely observed for 2 hours. If there was no ADR, observation was prolonged for 4 to 6 hours before allowing to leave our department. Phone contact was maintained for one week.
Statistics: Basic statistics (mean, standard deviation, median) were obtained from Microsoft Excel™ tables, Mann-Whitney test and Mc Nemar’s test by MedCalc™ vers.18.2.1 (Ostend Belgium) program was used.
Results
Out of 47 patients 19 men age 62.4 ± 9.1 and 28 women age 67.5 ± 12.1 were included. The pathologic condition which necessitated the present demand for using RCM were: Arterial 13(27.7%), pacemaker 1(2.1%), other vascular anomaly 5(10.6%), chest, abdominal CT 16(34.1), aneurysm 8(17.1%), vertebral fracture 1(2.1%) head-neck tumor 1(2.1%) fistulography 2(4.2%). summarizes the (supposed or documented) ADR emerging from the patients’ history with reference to the suspected culprit RCM, the type of ADR and the mean time elapsing between ADR event and our testing (Table 1). The majority of patients (59%) fell into the (IHS) group, but only 28 of 32 had definitively been diagnosed as anaphylaxis, urticaria or erythema occurring within the first hour after RCM administration. Similarly out of 11 patients with Delayed Hyper Sensitivity (DHS) symptoms manifesting after 60 minutes up to 7 days only 9 could be categorized due to clinical phenotypes. Nine patients could not clearly remember either the pathologic conditions or the intervals between receiving contrast media and onset of Adverse Event (AE). Additional 3 patients remembered severe and widespread skin symptoms after organic iodine application upon trauma or surgical interventions. There was an overlap amongst these categories, because some patients experienced more than one ADR. Two patients were included who had previously been exposed to internal radio-iodine treatment (column 5, line 5) and there was a concern regarding sensitization.
| Parameters | Total cases n=47 Diagnoses n=53 |
Immediate hypersensitivity n=32 (59.2%) |
Delayed hypersensitivity n=11(20.4%) |
General symptoms without skin involvement n=7(13.0%) |
Local iodine exposition induced severe skin symptoms n=3(5.5%) |
|---|---|---|---|---|---|
| Age yr ± S.D. | 65.1 ± 11.6 | 65.3 ± 12.1 | 67.1 ± 12.7 | 64.4 ± 9.8 | 59 ± 22.6 |
| Clinical manifestations from history and observed |
Anaphylaxis=13 Urticaria ± ANO=8 Local to generalized flushing ± pruritus or burning=7 |
MPE=5 Eczema=1 SDRIFE**=2 Psoriasis=1 (unrelated ) |
self-reported malaise |
no previous RCM exposition |
|
| Time elapsing until testing (yrs), SD, median | 13.8 ± 15.4 9.5 |
13.6 ± 13.7c 9.5 |
5.9 ± 10.2c 0.4 |
15.8 ± 7.1 17 |
15.3 ± 9.2 10 |
| Allergy in history verified or suspected | 40/47 (85%) | 28/40 (poscases) (70%) |
10/40 (25 %) | 2/40 (5%) | |
| Malignancy | 11/47 (23%) | 9/11 (83%) | 2/11 (17%) | 0 | 1/11** |
| History of previous culprit RCM | 23/4 (49%)a | 15/32 (47%) | 6/10 (60%) | 2/18 (11%) a internal iodine treatment | 1/18 (11%) a,b |
| Unknown history of hypersensitivity to RCM | 14/4 (30%)a | 9/24 (37%) | 3/10 (30 %) | ||
| Reported RCM | 23 (100%) | 17 (100%) | 6 (100%) | not known | not reported |
| Iohexol | 12/24 (52) | 7/17 (41.2) | 5/6 (83) | ||
| Ioversol | 2/24 (8.7) | 1/17 (5.9) | 1/6 (17) | ||
| Iomeprol | 2/24 (8.7) | 2/17 (11.8) | 0 | ||
| Iobitridol | 2/24 (8.7) | 2/17 (11.8) | 0 | ||
| Diatrizoic acid | 1/24 (4.3) | 1/17 (5.9) | 0 | ||
| Iopromid | 1/24 (4.3) | 1/17 (5.9) | 0 | ||
| Iothalmat | 1/24 (4.3) | 1/17 (5.9) | 0 | ||
| Iopamidol | 1/24 (4.3) | 1/17 ( 5.9) | 0 | ||
| Dimeglumingadopentat | 1/24 (4.3) | 1/17 (5.9) | 0 |
Note: Altogether 100%a; one of the “delayed “cases (Column 4, lines 1-3)b ; difference statistically significant c
Table 1: Characteristics of tested subjects.
The time elapsing between AE and our testing exceeded in average 13 years except for the cases with DHS and skin symptoms (5.9 ± 10.2 years). The difference against IHS was significant (p=0.033). Out of the total of 47 patients 34 reported over one year, whereas 13 within one year intervals. The majority of our cases 40/47(85%) reported various allergies (drugs, pollens, metal ions balm of Peru etc.) but only 23/47 (49%) mentioned RCM as previous culprit substances. Malignancies emerged in 23% of the patients necessitating repeated RCM-CT investigations with safe material(s). Most of the non-cancer cases needed vascular investigations (DSA or CTA including coronariography). One patient each was on chronic haemodyalysis and on regular plasma exchange therapy; both expected fistulography to be done. Our youngest female patient needed salpingography. The distribution of various RCM among the immediate and delayed HS cases showed that iohexol had emerged in the majority of cases, followed by ioversol. Iomeprol and iobitridol had only been suspected in conjunction with IHS. All others were less frequent, Meglumine gadopentetate is a Magnetic Resonance Imaging (MRI) contrast material that was not tested further. We were unable to categorize those 9 patients self-reporting generalized malaise due to (mostly) unknown Iodinated Contrast Media (ICM) in the past. Reliable medical documentation was missed in those cases as well. Kidney or brain involvement as toxic AEs could not be ruled out but was unlikely among our patients due to their history.
Study Design
The skin test results are summarized in Table 2. Nine materials were tested on different numbers of patients according to their personal history. The total tests performed in all cases were 123, i.e. 2.6 RCM were tested in average on each patient. The most frequently tested ones were iohexol followed by iopromid, iodixanol and iomeprol. These ICM made 87% of all tests/cases. The total tests in (116 SPT) were nearly identical with (118 IDT). One unusually late (48hrs.) appearing positive SPT was negative by IV. provocation. The frequency of positive test results was 2.8 times higher in the IDT as compared to SPT(Mc Nemar test p=0.0001). There were some variations, however, among the individual RCM results, iohexol gave the highest positivity rates in SPT and second highest in IDT (27% and 42.5%), whereas iomeprol at much lower tested patients’ numbers yielded the highest (45.4 %) IDT positivity rate. SPT positivity with iopromid was the lowest(6.3%). The LOD results were regarded as positive. The ratio of “only late” positivity in SPT(39%) was almost 5 times higher than that in IDT(8.1%) The majority of IDT positive results was achieved by the lower (10-3 m) or by both concentrations. Only 21.2% IDT positive results were exclusively due to the the higher (10-2m) concentration (Table 2). All skin tests were negative in ten patients. A comparison was made among the 70’ IDT-readings as related to the “standard” 20’ values. As seen in Figure 1, 38.2% of the positive values- obtained with various RCM in 34 cases- have shown increased diameters by 70’, while in 5.9% the positivity became visible only at the time of that second “early” reading. The remaining IDT diameters were partly unchanged (29.4%) or the positivity has faded (23.5%). There was one systemic AE directly associated with skin testing; a 48 year-old man suffering from sarcoidosis and RCM-related ANO was, unlike others, because of urgent need for results started testing with Iobitridol and Iomeprol could have been the culprits 3 years ago for ANO during CT. Iopromid, Iobitridol and Iomeprol became all positive at already 3-4 min. after injecting test materials and he collapsed then but has recovered within 5 minutes with no need for emergency treatment. Vital parameters were normal; no change either in blood pressure or in pulse rate has occurred. Serum tryptase was measured within one hour and after 24 hours; 4.5 and 5.0 mg/l (normal range 0.5-11 mg/l) values were obtained not suggestive for anaphylaxis. On the next day he tolerated IV provocation (DPT) with iohexol and after 5 consecutive days coronariography could be performed with this RCM without any side effects. Another patient (51 year-old women) was tested negative in both SPT and IDT series but has developed localized MPE after more than 16 hours on her hip. The tested substances were iohexol and iodixanol.
| Tested materials/cases | Prick neg. | Prick LODa | Prick pos. | Intraderm (ID) neg. | Intraderm LODa | Intraderm (ID) pos . | Intrad. only 10-2 mol pos. | Intraderm. only late pos. | Prick only late pos. | Prick not done | Prick total | Intraderm total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iohexol 45 | 30 | 2 | 7+5 late | 21 | 2 | 17 | Aug-17 | Feb-17 | 04-Dec | 1 | 44 | 40 | |
| Iopromid 34 | 26 | 2 | 1+1 LOD | 19 | 2 | 13 | 3 | 0 | 1 | 2 | 32 | 34 | |
| Iomeprol 11 | 8 | 0 | 1 only late |
5 | 0 | 5 | 1 | 2 | 1 | 0 | 11 | 11 | |
| Iobitridol 8 | 5 | 1 | 0 | 4 | 2 | 2 | 1 | 0 | 0 | 2 | 6 | 8 | |
| Iodixanol 17 | 12 | 2 | 2 | 10 | 2 | 5 | 01-May | 02-May | 01-Feb | 0 | 17 | 17 | |
| Meglumin Diatrizoat 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 bleeding not valid | 0 | 1 | |
| Ioversol 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| Diatrizoat 3 | 2 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 3 | |
| Ioxithalamate meglumin 3 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 2 | 3 | |
| All 123 | 86 | 7 | 11 + 7 late 18 | 63 | 8 | 46 | 14 | 6 | 7 | 7 | 116 | 118 | |
| Ratio of positive tests % | Ratio of LOD tests % 7/18= 39% | Ratio of+tests % 18/11= 16% | Ratio of LOD test % 8/74= 10.8 % | 46/66=69.7% 10-3 m only; or +10-2 m concentr. | 14/66=21.2% 10-2 m only concentr. | 6/66=8.1% | 7/18=39% | 25/11=22.3%* | 74/11= 62.7%* | ||||
Note: Limit of Detection a; difference between Prick and ID positivity *p<0.0001
Table 2: Skin test results.
Figure 1: Percentual distribution of changes in ID test-size at 70 minutes in relation to 20 minutes readings D: Bleb diameter, Columns 3+4 (44.1%) mark changes toward positivity. Column 5 (one case) refers to unusual appearance of skin test positivity.
Within the IHS group (35 cases) we detected 44“early” and 14 late positivity. The total number of cross-reactive cases was 16. Out of 35, seven patients were all negative, and an additional person had ID “Limit of Detection” (LOD) value. This together with 2 others has been proven positive while performing DPTs. Three others had been false positive as DPT was negative. The analysis of DHS group has revealed similar values. These 11 cases showed in the “early reading” phase 13 accepted positivity (in 7 cases) and only 3 by the “late” evaluation. In this group 4 cases had only negative tests. False negative results were absent but two false positives were noted. The cross-reactivity was in DHS slightly lower than in the IHS group; 5/10(50%) as contrasted to 16/28(57%). These numbers are meant after correction of for false positive and false negative results. Together the IHS plus DHS group contained 11 cases out of 47 with only negative tests (23.4%). In conclusion, the decision which RCM should be used in DPT and used later on was based mostly on ID test results. Some clinical examples are demonstrated in (Figure 2). Some difficulties arising with proper evaluation of tests are obvious.
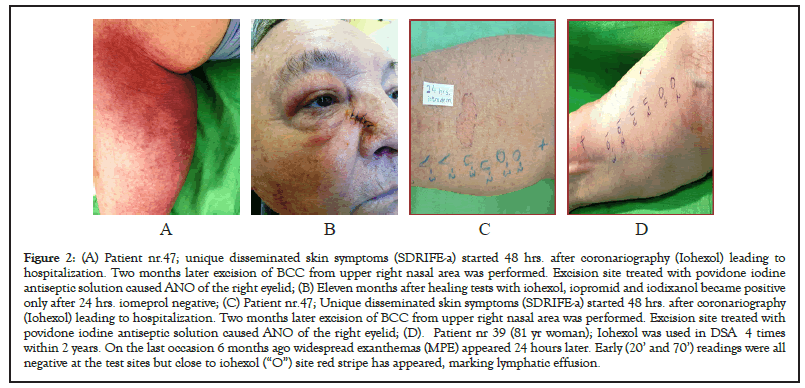
Figure 2: (A) Patient nr.47; unique disseminated skin symptoms (SDRIFE-a) started 48 hrs. after coronariography (Iohexol) leading to hospitalization. Two months later excision of BCC from upper right nasal area was performed. Excision site treated with povidone iodine antiseptic solution caused ANO of the right eyelid; (B) Eleven months after healing tests with iohexol, iopromid and iodixanol became positive only after 24 hrs. iomeprol negative; (C) Patient nr.47; Unique disseminated skin symptoms (SDRIFE-a) started 48 hrs. after coronariography (Iohexol) leading to hospitalization. Two months later excision of BCC from upper right nasal area was performed. Excision site treated with povidone iodine antiseptic solution caused ANO of the right eyelid; (D). Patient nr 39 (81 yr woman); Iohexol was used in DSA 4 times within 2 years. On the last occasion 6 months ago widespread exanthemas (MPE) appeared 24 hours later. Early (20’ and 70’) readings were all negative at the test sites but close to iohexol (“O”) site red stripe has appeared, marking lymphatic effusion.
Intravenous Drug Provocation (DPT) results
Out of 47 patients 38 underwent DPT resuming. The tested RCM were iohexol (10; -2 positive), iopromid (10; –all negative), iodixanol (9; -3 positive) iobitridol (5; -1 positive). Lysin-amidotrizoate and iomeprol (2 each; -none positive). Thus, results accounted for 6 additional positive tests/cases in addition to skin testing, i.e. 15.4 % overall positivity increase over skin tests among 38 patients (Table 3). The AEs due to DPT were all mild with skin symptoms within 24 hours (2 cases – one of them Figure 3) or developed minor changes in vital parameters, like transient drop in blood pressure or nasal obstruction as well as circumscribed MPE (after 15 mins–16 hours). In none of them was any intervention necessary. DPT was not performed in 9 cases; 2 were excluded because skin tests with 3-4 various materials were all positive. One of them had been skin tested during allergy season and had documented strong ragweed allergy as well. Because of her adherence on RCM testing we have repeated skin tests including iobitridol as new, 3 months later after pollens ceased and obtained iobitridol IDT negativity followed by its tolerance in DPT. Subsequently the patient underwent successful RCM-CT examination. The other, a man (Figure 2a) has rejected DPT. Two months after widespread skin eruption (SDRIFE –Figure 2a) due to RCM he developed periocular ANO after an antiseptic iodine containing solution (Figure 2b). All skin tests became positive only after 24 hours (Figure 2c). Out of the remaining seven cases 3 had been skin tested by ioxithalamate meglumin which is for oral/anal use only (Figure 2d). The remaining 4 patients had all tolerated in real life the ICM selected upon skin test negativity within 1-2 month after our testing. Patients were released on the third day with final recommendations for the future RCM to use.
| Tested materials | Positive | Negative | Total | Positive (%) |
|---|---|---|---|---|
| Iohexol | 2 | 8 | 10 | 20 |
| Iopromid | 0 | 10 | 10 | 0 |
| Iodixanol | 3 | 6 | 9 | 33 |
| Lysin amidotrizoate | 0 | 2 | 2 | 0 |
| Iobitridol | 1 | 4 | 5 | 20 |
| Iomeprol | 0 | 2 | 2 | 0 |
| Tests together | 6 | 32 | 38 | additional 15.8 |
| Not tested(out of 47)patients* | - | - | 7 | 14.9 |
| Provocation refused because all skin tests were positive** | - | - | 2 | 4.3 |
Note: *No provocation was performed with ioversol, meglumin diatrizoate and ioxithalamate meglumin; **One of them was successfully (negatively) retested by iobitridol out of pollen season and has tolerated it upon systemic administration
Table 3: Summary of intravenous provocation tests (Step 3).
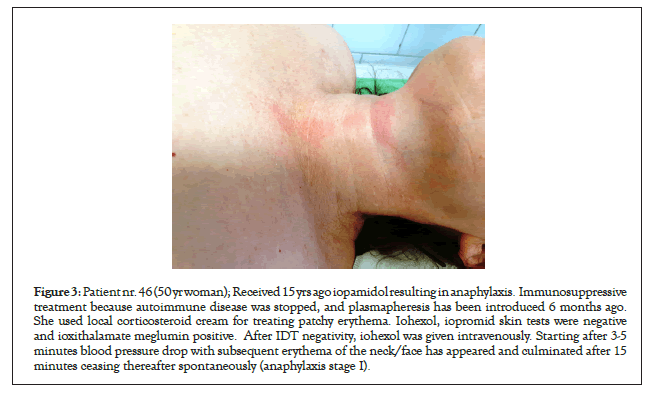
Figure 3: Patient nr. 46 (50 yr woman); Received 15 yrs ago iopamidol resulting in anaphylaxis. Immunosuppressive treatment because autoimmune disease was stopped, and plasmapheresis has been introduced 6 months ago. She used local corticosteroid cream for treating patchy erythema. Iohexol, iopromid skin tests were negative and ioxithalamate meglumin positive. After IDT negativity, iohexol was given intravenously. Starting after 3-5 minutes blood pressure drop with subsequent erythema of the neck/face has appeared and culminated after 15 minutes ceasing thereafter spontaneously (anaphylaxis stage I).
Fate of the tested patients – prospective assessment of (real life) events
The below table summarizes the data obtained in succession after the 3 steps had been finished. We examined 46 patients’ “real life” outcomes and found that 39 had no problem with later use of RCM that has been selected. In 35 cases (74.5%) the next radio contrast-assisted CT, arteriography or DSA was done in the same calendar year usually 5 to 90 days after the patients’ release from our department. Four patients did not undergo repeated imaging procedure. Mostly because of temporary shortage of recommended RCM at the investigation site. On the other hand, radiologists did not always obey our recommendation or preferred premedication instead. This was found by tracking histories in 13 cases. One of them developed skin rash (Column 4, line 6). Out of the 9 patients who were tested but up till now without investigational event, 6 are scheduled to that (Column 6). In some patients more than one investigation was carried out subsequently. Taking together iomeprol was the safest alternative. Iohexol and Iodixanol were more dangerous. To our present knowledge 3 patients have died (2 to 5 years after testing) because of progressive malignant tumors and heart attack, respectively. At least 4 had left our hospital equipped with the results but their fate remained obscure for us. These figures show that 84.8% of all 3-Step tested patients were able to receive a useful alternative RCM-assisted diagnostic intervention to their benefits.
Discussion
The hypersensitivity concept as background for unpredictable AE to RCM emerged in the first decade of this century [3-6]. Except for toxicity it might be difficult to differentiate HS reactions from those that are unrelated to previous RCM administration. These were categorized as mild (e.g. limited nausea, headache, transient flushing, vasovagal reaction that resolved spontaneously), moderate (e.g. hypertensive urgency, isolated chest pain or vasovagal reaction that required treatment) and severe (e.g. arrhythmia, convulsions, hypertensive emergency and treatment-resistant vasovagal reaction). The HS-related “allergic-like” reactions were also subdivided according to the above categories as outlined in Table 1[8]. Both the treatment of the AEs and the pretreatment to avoid them in selected patients expecting repeatedly RCM had been described in the US guidelines [8]. Even with corticosteroid plus antihistamine premedication 16.7% overall recurrence was noted in earlier mild but less frequently in severe reactors [9]. In our studies we concentrated to immediate and delayed HS events but a considerable uncertainty and overlap with RCM-unrelated and non-immune mediated ones had to be considered (Table 1). In a 61 years old patient with anaphylaxis to iohexol and positive DPT to iodixanol recurrence-free reintroduction with corticosteroid and H1 antihistamine pretreatment could be performed (Table 4). Recent studies stated that the allergic background in IHS reactions was at least 21% or higher and its frequency had increased with AE severity up to 100% manifesting in cardiac arrest [10]. We were able to differentiate the main phenotypes of IHS from DHS upon individual history and by medical records. Facial/neck flushing is an important early sign that we observed in our patient after positive DPT (Fig 3). The DHS group was less represented; 11 against 32 IHS suspected (9 and 28 classified-(Table 1)). Similar results had been reported in smaller clinical case series (6 DHS against 17 IHS-ref 11) [6-12]. Within DHS cases we diagnosed 2 with SDRIFE starting at 1-3 days after RCM administration. Both required 7-10 days hospitalization until resolution. SPT and IDT have revealed only late reactions (positivity at 24 hrs) with more tested RCM solutions (i.e. cross-reactivity). Both of them were men and one had received after negative IDT Iobitridol IV with negative DPT result, while the other has refused further testing. This man had been treated 32 years ago by oral potassium iodide and 2 months after SDRIFE he experienced ANO due to organic iodine antiseptic solution. SDRIFE known for dermatologists for decades (as “baboon syndrome”) has been described in connection with RCM in only less than 10 cases [13-14]. An earlier study concluded that molecular 131Iodine thyroid treatment in 7000 cases had not caused any subsequent HS reaction in RCM assisted CT investigation [15]. In two other patients’ history widespread eczematous rashes to local iodine containing creams (but no prior AE to RCM) could be revealed years before testing with RCM Table 1, Col. 6 Both were negatively skin tested by various RCM and tolerated DPT as well. The concentration of skin tests is an important issue. In an ENDA/EAACI position paper the nonirritating concentrations were recommended for many drugs including ICM [16]. For SPT undiluted RCM and for ID 1/10 dilutions have been proposed. More recent information concluded that undiluted RCM might be used ID for mild cases but the optimal concentration in general is not established yet [11]. Our experience with various drugs (antibiotics, NSAIDs, local anesthetics) led to the conclusion that after AEs of allergic origin the clinical phenotypes observed together with the timing were of utmost importance and a uniformly set safe concentration of 10-3 m would abolish the differences originating from the different chemical composition [17]. This corresponded for most nonionic RCM around 1/400 dilution, except for iodixanol (dimeric CM), where the dilution was 1/200. Taking into consideration the recommended 1/10 dilution for IDT all our skin testing was performed with 10-2 m solutions (1/40) as well. Even using those higher dilutions one polysensitized patient collapsed. Within our entire IDT series, the ratio of positivity occurring exclusively at 10-2 m solutions was 14/66 (21.2%) . Paradoxically, most cases were positive to 10-3 m solutions or to both ones. The observed phenomena could be attributed partly to hapten binding to receptor, thus to pharmacological interaction (p-i) of high specificity described by Pichler [18]. Only 6/66(8.1%) tests were positive exclusively by late readings if LOD excluded (Table 2). The positive IDT results were obtained far beyond one year after the AEs opposite to general opinion that argues for optimal testing within 6 months after AE [9-12]. Surprisingly, the low concentrations used in IDT were active in demonstrating 62.7% positivity rate exceeding 2.8 times the SPT average positivity which has occurred with undiluted media that might have caused bleeding as sign of local toxicity (Table 2). The skin test positivity within the suspect population varies widely between 4.2 and 73% according to literature. A Spanish study ended up at only 7.6% using 1/10 dilutions but neither do not second “early” reading at 70’ nor late reading at 24 hours was done [19]. The guidelines mentioned only one compulsory reading time for IDT, namely 20 min [20]. We made in addition a second reading at 70’ and found in almost half of the cases a further increase in the bleb Diameters (D) with evolving positivity in 2 cases exclusively at this time point (Figure 1). These would have been marked “negative” without the second checking. Of interest could be our observation with almost no reactivity at the test site but a red stripe marking lymphatic effusion (Figure 1d) which could mark starting systematization of the positive reaction. Similarly, but with no visible local reactions in IDT, DHS developed many hours later in one patient but we may not have known which of the two tested substances iohexol or iodixanol (or both) were responsible (Figure 1). The DPT is considered as gold standard. In agreement with the consensus our testing was aimed to verify the selection of an alternative RCM indicated by cumulated skin test results [21]. In an earlier study only DHS cases were tested first by IDT, followed by DPTs. ID tests after undiluted or 1/10 diluted RCMs have been red at 20’ only and afterwards on 1-3 consecutive days. DPTs were done using saline diluted RCM and stepwise increased concentrations starting at 1/100 in successive manner. Out of 127 skin test negative patients 44 (34.6%) became positive [22].
| RCM as recommended after 3 Steps | No problem | Done in the same calendar year | Adverse event | DPT ignored | Next, not performed yet | Reasons | All within 1 year | Not within 1 year |
|---|---|---|---|---|---|---|---|---|
| Ioversol | 1 | 0 | 0 | 0 | 0 | 1 | 0 | |
| Iohexol | 10 | 12 | 2 | 5 | 3 | 15 | 1 | |
| Iomeprol | 2 | 1 | 0 | 1 | 1 | 1 denied | 3 | 0 |
| Iodixanol | 11 | 11 | 2 | 4a | 0 | 1 with premedication successful | 11 | 0 |
| Iobitridol | 4 | 2 | 1b | 1 | 2 | 4 | 0 | |
| Diatrizoat | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |
| Iopromid | 11 | 8 | 0 | 2 | 3c | 11 | 3 | |
| All together | 39 | 35 | 5 | 13 | 9 | 2 | 46 | 4 |
Note: Material not available at X-ray investigation site, but alternative choice upon skin test negativitya; Performed successfully with premedication: Mild skin symptoms after 36 hrsb; No urgent demand for performing RCM-CT
Table 4: “Real life” outcomes after 3 step testing.
Two more recent publications has not found any positive DPT after testing 22 IHS patients with negative skin tests to the suspected RCM or 18 DPT in a “mixed” patient group containing non-immune, IHS and DHS reactors who were skin test negative to alternative RCM [20- 22]. Our results are between those extremes (0 to 35%) and indicated 15.4% additional positive results. We could confirm the highest DPT positivity rate of iodixanol followed by iohexol i.e. of two closely related compounds. In those cases in which diatrizoates (earlier used ionic RCM) emerged from history a rational alternative for skin testing was ioxithalamate meglumin which gave 2 positive ID results out of 3 tests. Other nonionic RCM tests were all negative. As ionic CM are nowadays of limited use, the verification by DPTs was abandoned. The cross reactivity among our tested patients was slightly higher (50 to 57%) than in a much larger cohort with more tested RCM [23]. Dermatoallergist’s experience properly evaluating skin tests and DPT was necessary. We share the view stopping local corticosteroids at least 2 weeks before skin testing because this might have led to false negative IDT in the case shown in (Figure 3) [7].Searching databases, we could not find any information dealing with the “real life” outcomes on RCM tested patients; the negative predictive value for the skin tests was published though [24]. Therefore, we feel that the translational data summed up in are unique and enable to draw a positive conclusion on our 3-Step investigational protocol (Table 4). In conclusion we greet the opinion expressed by the Italian radiologists and allergists that a better collaboration is needed between these fields extended also to involvement of the national/international pharmacovigilance services [25].
Conclusion
“Better safe than sorry” was the concept behind these studies. This prompted us to hospitalize most patients for some parts of tests. Testing is the only reasonable alternative to help those patients with uncertainty/allergy due to previously used iodinated contrast media for computer tomography. Premedication without testing should not be practiced. The three-step workup shown in a single-center based study was proven useful as reflected by the near 85% versatility within the next year.
Acknowledgements
Authors are indebted to Elisabeth Pintér, M.D. for her help in statistics and to Csaba Sükösd PhD for his help to finalize manuscript.
Author Contribution
B-BJM conceived the study, carried out all provocation tests, participated in skin testing and drafted the manuscript. KM and K-KA participated in clinical work, test reading, data collection and corrections. VA selected the patients and helped in writing manuscript.
Data Availability Statement
All datasets generated for this study are appearing in the article. Some results especially concerning the “real life” considerations are stored in the personal patient records.
Ethics Approval
The skin testing for detection of a possible allergic/irritative reaction to a drug does not require authorization by local ethical committee. The complete study, however, has been approved by the ethical committee (RKEB) under the title: “Investigation into the personal tolerability of various X-ray contrast materials”. The patients provided their written informed consent to participate in this study which is stored with the personal files.
Conflict of Interest
Authors have no conflict of interest.
Funding
None
REFERENCES
- Andreucci M, Faga T, Serra R, De Sarro G, Michael A. Update on the renal toxicity of iodinated contrast drugs used in clinical medicine. Drug Healthc Patient Saf. 2017;9:25-37.
- Yang Z, Rong L, Yue J, Wei Y, Zhang X, Yin R. Fatal contrast medium-induced adverse response to iohexol in carotid artery angioplasty. Medicine. 2019;98(33):e16758.
- Brockow K, Romano A, Aberer W, Bircher AJ, Barbaud A, Bonadonna P, et al. Skin testing in patients with hypersensitivity reactions to iodinated contrast media – a European multicenter study. Allergy 2009;64(2):234-41.
- Böhm I, Nairz K, Morelli JN, Keller PS, Heverhagen JT. Iodinated contrast media and the alleged "iodine allergy": An inexact diagnosis leading to inferior radiologic management and adverse drug reactions. Rofo. 2020;124(5):451-8.
- Brockow K, Christiansen C, Kanny G, Clément O, Barbaud A, Bircher A, et al. Management of hypersensitivity reactions to iodinated contrast media. Allergy. 2019;60(2):150-8.
- Kanny G, Pichler W, Morisset M, Franck P, Marie B, Kohler C, et al. T-cell mediated reactions to iodinated contrast media: evaluation by skin and lymphocyte activation tests. J Allergy Clin Immunol. 2005;115(1):179-85.
- Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy. 2021;57(1):45-51.
- ACR Manual on Contrast Media Version 10.3 USA (2018).
- Kim SH, Lee SH, Kang HR, Lee SM, Park HW, Kim SS et al: Outcomes of premedication for non-ionic radio-contrast media hypersensitivity reactions in Korea. Eur J Radiol 2011;80(2):363-7.
- Clement O, Dewachter P, Mouton-Faivre C, Nevoret C, Guilloux L, Bloch Morot E, et al. Immediate hypersensitivity to contrast agents: The French 5-year CIRTACI Study. EClinical Medicine 2018;1:51-61.
- Ahn YH, Koh YI, Kim JH, Ban GY, Lee YK, Hong GN, et al. The potential utility of iodinated contrast media (ICM) skin testing in patients with ICM hypersensitivity. J Korean Med Sci. 2015;30(3):245-51.
- Rosado Ingelmo A, Doña Diaz I, Cabañas Moreno R, Moya Quesada MC, García-Avilés C, García Nuñez I, et al. Clinical Practice Guidelines for Diagnosis and Management of Hypersensitivity Reactions to Contrast Media. J Investig Allergol Clin Immunol. 2016;26(3):144-55.
- Arnold AW, Hausermann P, Bach S, Bircher AJ. Recurrent flexural exanthema (SDRIFE or baboon syndrome) after administration of two different iodinated radio contrast media. Dermatology. 2007;214(1):89-93.
- Huynh T, Hughey LC, McKay K, Carney C, Sami N. Systemic drug-related intertriginous and flexural exanthema from radio contrast media: A series of 3 cases. JAAD Case Rep. 2015;1(3):147-9.
- Konrády A. Iodine allergy-adverse reactions to contrast media. Orv Hetil. 2015;147(10):469-72.
- Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, et al. Skin test concentrations for systemically administered drugs -- an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013;68(6):702-12.
- Baló-Banga JM, Vajda A. Attempts to standardize intradermal drug tests based on molecular mass and on clinical phenotypes. Some pitfalls or exceptions. Translational Allergy. 2014;4(3):702-12.
- Yun J, Cai F, Lee FJ, Pichler WJ. T-cell-mediated drug hypersensitivity: Immune mechanisms and their clinical relevance. Asia Pac Allergy. 2016;6(2):77-89.
- Morales-Cabeza C, Roa-Medellín D, Torrado I, De Barrio M, Fernández-Álvarez C, Montes-Aceñero JF, et al. Immediate reactions to iodinated contrast media. Ann Allergy Asthma Immunol. 2017;119(6):553-557.
- Trautmann A, Brockow K, Behle V, Stoevesandt J. Radiocontrast Media Hypersensitivity: Skin Testing Differentiates Allergy From Nonallergic Reactions and Identifies a Safe Alternative as Proven by Intravenous Provocation. J Allergy Clin Immunol Pract. 2019;7(7):2218-24..
- Barbaud A, Weinborn M, Garvey LH, Testi S, Kvedariene V, Bavbek S, et al. Intradermal Tests With Drugs: An Approach to Standardization. Front Med (Lausanne). 2020;7:156.
- Torres MJ, Gomez F, Doña I, Rosado A, Mayorga C, Garcia I, et al. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy. 2012;67(7):929-35.
- Kwon OY, Lee JH, Park SY, Seo B, Won HK, Kang Y, et al. Novel Strategy for the Prevention of Recurrent Hypersensitivity Reactions to Radiocontrast Media Based on Skin Testing. J Allergy Clin Immunol Pract. 2019;7(8):2707-13.
- Schrijvers R, Breynaert C, Ahmedali Y, Bourrain JL, Demoly P, Chiriac AM, et al. Skin Testing for Suspected Iodinated Contrast Media Hypersensitivity. J Allergy Clin Immunol Pract. 2018;6(4):1246-54.
- Costantino MT, Romanini L, Gaeta F, Stacul F, Valluzzi RL, Passamonti M, et al. SIRM-SIAAIC consensus, an Italian document on management of patients at risk of hypersensitivity reactions to contrast media. Clin Mol Allergy. 2018;18:13.
Citation: Baló-Banga JM, Kádas M, Kása-Kovács A, Vajda A (2021) Three-Step Diagnostic Assessment Following Adverse Reaction due to X-ray Contrast Media: An 11-Year Study in 47 Patients. J Allergy Ther. 12:261.
Copyright: © 2021 Baló-Banga JM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.