Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2021)
Background: COVID-19 continuous spreads and causes numerous challenges for health care system. Vitamin D modulates the immune system by different mechanisms.
Objective: To examine the effect of vitamin D status of hospitalized COVID-19 patients and its influence on the severity of disease.
Subjects and methods: A retrospective multicentre cross-sectional study was conducted in the Western region of Saudi Arabia (SA) between June and August 2020. The demographic and clinical characteristics, laboratory tests included serum 25(OH)D and clinical outcome included admission for the intensive care unit (ICU), length stay on hospital, mechanical ventilation (MV) support and mortality were recorded and analysis for 197 of COVID-19.
Results: 144 (73.10%) had serum 25(OH)D<20 ng/ml, 31 (15.74%) had serum 25(OH)D ≥ 20 ng/ml and 22(11.17) had >30 ng/ml. 119 (60%) were discharge with mean serum 25(OH)D 18.98 ± 1.12 ng/ml, 56 (28%) were hospitalized with mean serum 25(OH)D 13.23 ± 0.97 ng/ml and 22 (11%) were deceased with mean serum 25(OH)D 16.20 ± 2.41 P=0.02. After adjusted covariance’s such as age, gender, diabetes, hypertension and chronic kidney disease (CKD), multiple logistic regression reveals intensive care unit (ICU) admission (Odd Ratio, OR 1.25 (95% confidence interval, CI, 0.41-3.88) P=0.70), mechanical ventilation (MV) support (Odd Ratio, OR 3.12 (95% confidence interval, CI 0.74-13.21) p=0.12) and mortality (Odd Ratio, OR 2.39 (95% confidence interval, CI 0.31- 18.11), p=0.40) weren’t significant among COVID-19 patients.
Conclusion: these data didn’t support the association between serum 25(OH) D and severity of the disease among hospitalized COVID-19 patients.
Coronavirus; COVID-19; Vitamin D; Infection; Immunity
A series of pneumonia cases caused by a novel coronavirus (2019- nCOV) was identified in Wuhan, China, at the end of 2019 [1]. The virus was renamed Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by the International Committee on the Taxonomy of Viruses (ICTV) [2]. The disease caused by SARS-CoV-2 was officially named COVID-19 by the World Health Organization (WHO) on February 11, 2020 [3]. The WHO classified COVID-19 as a global pandemic and a public health emergency on March 11, 2020 [4]. Since the first case of COVID-19 was recorded, researchers have been attempting to understand the mechanism of this virus and how to prevent and mitigate its symptoms. One possible method is augmenting vitamin D levels. Some studies have shown that a high level of vitamin D is associated with a lower severity of symptoms [5,6].
COVID-19 and its complications
The first case of COVID-19 was reported in Saudi Arabia on March 2, 2020. At the time of writing, there have been 329,271 cases and 4,458 deaths recorded in Saudi Arabia [7]. Minimizing the rate of SARS-COV2 infection and the severity of COVID-19 symptoms is crucial to reducing the burden on the healthcare system. The clinical manifestations of COVID-19 vary from asymptomatic to life-threatening conditions [8]. The most common symptoms of COVID-19 are fever, fatigue, myalgia, and coughing. Less common symptoms are headache, runny nose, sputum production, sore throat, hemoptysis, diarrhea, and sore throat [1].
Patients with severe symptoms require hospital admission and may develop to complications such as acute respiratory distress syndrome (ARDS), acute respiratory injury, renal injury, arrhythmia, and septic shock, requiring ICU therapy [9,10].
Approximately 40% of hospitalized patients with COVID-19 developed ARDS, and more than 50% eventually died [11]. Severe cases of COVID-19 progressed to ARDS rapidly, within nine days [1]. In addition, COVID-19 patients with ARDS may develop to multi-organ dysfunction even in younger adults or in those without comorbidities [12].
The mortality rate has increased among elderly male COVID-19 patients with comorbidities such as hypertension, diabetes, and obesity, or patients in specific ethnic groups, specifically Black, Asian or minority ethnic (BAME). All these factors are associated with vitamin D deficiency [13].
Vitamin D levels and the severity of COVID-19 symptoms
Vitamin D plays a pivotal role in the immune system. Vitamin D receptors (VDR) express in many types of cells, including immune cells such as T cells, B cells, dendritic cells, and macrophages [14]. Moreover, vitamin D has several immunomodulation effects against viral infection [15]. It enhances the innate immune system through the production of human antimicrobial peptides, such as cathelicidin and B-defensin, that lowers the rate of viral infection [16].
However, vitamin D’s role in combating viral infection through macrophage defense is related to the cytokine response rather than killing the virus [17]. Moreover, it suppresses the cytokine storm by preventing the excessive production of pro-inflammatory cytokines [18]. Additionally, vitamin D modulates the adaptive immune system by suppressing the secretion of pro-inflammatory cytokines mediated by type-1 T helper cells (Th1), such as interleukin 2 (IL2), interferon gamma (IFN ) and tumor necrosis factor (TNF), while it stimulates the production of anti-inflammatory cytokines mediated by type-2 T helper cells (Th2) [19,20]. Also, vitamin D is important in recruiting immune cells to infection sites and strengthening the junction integrity of epithelial cells [16]. In addition, vitamin D regulates renin-angiotensin system (RAS) by increasing the ratio of ACE2/ACE that leads to decreasing angiotensin 2 and enhancing the ACE2/Ang (1-7)/MasR axis consequently, reducing the production of inflammatory cytokines and the risk of lung injury [13,21].
Several observational studies suggest an association between vitamin D and COVID-19. An ecological study conducted by Ilie et al. found an inverse correlation between the mean value of vitamin D status for 20 European countries with the number of cases and the mortality rate of COVID-19, particularly for aging populations in Spain, Italy, and Switzerland [5]. Similarly, Merzon et al. conducted a large population base study that included 7,807 subjects recruited from the Leumit Health Services (LHS) database [6]. These participants underwent a COVID-19 test and had a plasma level of 25(OH)D measured during the study period. After controlling confounder factors such as age, gender, socioeconomic status, presence of mental or other chronic disease by multi-variant analysis, they found a positive association between a low serum level of 25(OH)D and confirmed cases of COVID-19 [6].
Although these studies suggest the role of vitamin D in COVID-19 infection, they have numerous weaknesses and limitations that affect the accuracy of the result. The data in the study conducted by Ilie et al. were heterogeneous, owing to the number of tests performed in different countries [5]. Moreover, the mortality rate was influenced by the different management approaches used by different countries. However, the study of Merzon et al. had a large sample size and multi-variant analysis for controlling confounder factors, but it did not assess the vitamin D level upon admission, symptoms, and clinical outcomes, as it was a database study [6].
Furthermore a retrospective observational study conducted by Carpagnano et al. in the Hospital Polyclinic of Bari, Italy [22]. That study investigated the possible association between the vitamin D level and severity of COVID-19 among 42 patients. Patients were classified into four groups based on serum vitamin D levels: normal (≥ 30 ng/mL), insufficient (20-29 ng/ml), moderate deficiency (10- 19 ng/ml), and severe deficiency (<10 ng/ml). They demonstrated that most COVID-19 patients (81%) had hypovitaminosis D (vitamin D<30 ng/mL). Moreover, they found an elevated level of C-reactive protein (CRP), ferritin, and D-dimer among all groups. IL6 was likely to be higher among the group with a severe deficiency of vitamin D, but the result was not statistically significant. Additionally, they reported a mortality risk significantly higher among COVID-19 patients with vitamin D deficiency than in other groups [22]. Although the study was well-designed by measuring confounder factors such as age, gender, BMI, smoker status, and comorbidities as well as measuring the serum vitamin D level during hospitalization, the results were affected by statistical error owing to a lack of power calculation and the small sample size.
In contrast, Panagiotou et al. conducted a retrospective analysis of 134 COVID-19 patients in Newcastle upon Tyne Hospital (NuTH) in the UK [23]. The serum 25 (HO)D for COVID-19 patients was measured upon admission. They found that patients in the ICU had a lower 25(OH)D level (33.5 nmol/L ± 16.8) than did non-ICU patients (48 nmol/L ± 38.2). Also, they found that vitamin D level was not associated with increased oxygen requirement, presence of comorbidities, CRP level, or NEWS-2 score.
Panagiotou et al. reported that 94 patients were discharged, 16 patients died, and 24 patients were still hospitalized [23]. However, they demonstrated that serum 25(OH)D was not associated with mortality [23]. Although this study was the first study measuring the serum vitamin D level among COVID-19 patients, the result might have been affected by a statistical error and the small number of participants. In addition, the confounder factors were not controlled, so the result is highly affected.
A recent pilot study of a clinical trial conducted by Entrenas Castillo et al. investigated the effect of calcifediol, in combination with hydroxychloroquine, azithromycin, and antibiotic (ceftriaxone), related to the needs of COVID-19 patients for ICU admission and the mortality rate [24]. The intervention group consisted of 50 patients who received calcifediol combined with the best available therapies, while the control group consisted of 26 patients who did not receive calcifediol. The laboratory analysis, respiratory test, and comorbidity rates were obtained for both groups. After the confounders, such as hypertension and diabetes, were adjusted for multivariate regression analysis, they demonstrated that vitamin D significantly reduced the need for ICU treatment. They found that 98% of patients treated with calcifediol did not require ICU admission and none died, while 50% of patients did not treat with calcifediol were admitted to the ICU, and two patients died [24]. Despite it was the first randomized control trial (RCT) to investigate this topic. The researchers did not measure the serum vitamin D level before starting the intervention. It may be useful as an indicator for the effects of vitamin D status on the severity of COVID-19 symptoms. It might also be helpful to individualize the doses of vitamin D based on the serum level to be more effective. Another limitation in this study was that the researcher did not assess body mass index (BMI), as obesity is a risk factor for both COVID-19 and vitamin D deficiency.
The scientific evidence related to the role of vitamin D in COVID-19 treatment is currently limited. The aim of this study to investigate the impact of vitamin D status on the severity of hospitalized COVID-19 patients in the term of ICU admission, MV support, length of hospital stay and mortality in Western region of SA.
Study design and population
A retrospective, observationl multicentred study was conducted in the Western region of SA. Based on a statistical power of 80%, confidence level 95%, margin of error 5% a minimum sample size of 197 COVID-19 was included. Both genders and aged 18 years and above were recruited in the study as the following: from King Faisal Hospital in Makkah (N=129), from Al Noor Specialist Hospital (N=44) in Makkah and from Complex King Faisal Hospital (N=24) in Taif (Figure 1). The study was approved by the institutional review board (IRB) in Taif region with approval number 377.
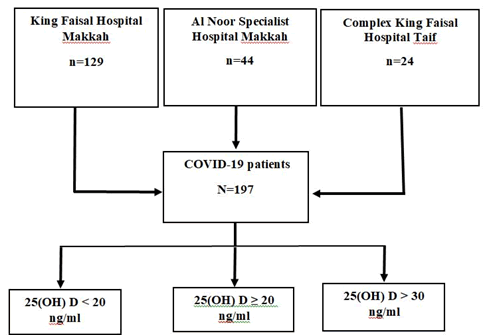
Figure 1: Algorithm for patient's recruitment to study.
Data collection
The data of total 197 COVID-19 patients were obtained from their files between June and August, 2020. The data collection included the hospital name and admission ward, gender, age, nationality, comorbidities, complications, length on hospital stay as well as severity of disease in term of oxygen and MV support, ICU admission and mortality.
Laboratory measurements
The serum vitamin D level was measure for all COVID-19 patients during hospitalization by using ADVIA Centaur XPT immunoassay system and vitamin D kit in king Faisal Hospital and Al Noor Specialist Hospital while using Cobos 6000 immunoassay with a Roch vitamin D kit in Complex King Faisal Hospital. The vitamin D status for patients was determined based on their serum 25(OH) D levels, according to the local recommendations to diagnosis and treatment vitamin of vitamin D deficiency. The patients’ vitamin D status was classified as the follows: adequate, 25(OH)D>30 ng/ ml; sufficient, 25(OH)D ≥ 20 ng/ml; and deficient, 25(OH)D<20 ng/ml (Figure 1) [25]. Other laboratory parameters were obtained from patient’s files include complete blood count (CBC), kidney function such as blood urea nitrogen (BUN) and creatinine, liver function such as alanine aminotransferase (ALA) and aspartate aminotransferase (AST), C-reactive protein (CRP) and serum vitamin D (25 OH).
Statistical analysis
Data analysis was performed using Statistical Package for the Social Sciences (SPSS) (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp). Frequency and percentages were used to display categorical variables, while means and standard deviations were used to display continuous variables. Chi square test and fisher exact test were used to test for the presence of association between vitamin D level and categorical variables. Shapiro-Wailk test and histogram chart were used to display a distribution of data. Square root logarithm equations were used to transfer data for normally distributed. Pearson correlation was used to assess the association between serum vitamin D and length on hospital stay as well as serum CRP. Post hock test was used to determine the least significant difference (LSD) on clinical outcome of COVID-19 patients based on serum vitamin D. Multivariable logistic regression was performed to determine the association between serum vitamin D and categorical variables with adjusting confounders such as age, gender and diabetes mellitus type 2 (DM), hypertension and chronic kidney disease (CKD). The level of significance was set at 0.05.
The baseline characteristics of COVID-19 patients are shown in Table 1. A total of 197 patients were included in the study. Among them, 47.72% were Saudi and 52.28% were non-Saudi. The vast majority of the patients were recruited from King Faisal Hospital in Makkah 129 (65.48%) followed by Al Noor Specialist Hospital in Makkah 44 (22.34%) and Complex King Faisal Hospital in Taif 24 (12.18%). 109 (55%) patients were admitted to general ward while 88 (45%) patients were admitted to ICU (Figure 2). Patients were 67.51% male and 32.49% female. The mean age of patients was 57.26 years.
Table 1: Demographic characteristics of study population.
| Demographic characteristics | N (%) |
|---|---|
| Hospital admission | |
| King Faisal Hospital in Makkah | 129 (65.48%) |
| Al- Noor Specialist Hospital in Makkah | 44 (22.34%) |
| Complex King Faisal Hospital in Taif | 24 (12.18%) |
| Ward admission | |
| General Ward | 109 (55 %) |
| ICU | 88 (45%) |
| Nationality | |
| Saudi | 94 (47.72%) |
| Non- Saudi | 103 (52.28%) |
| Gender | |
| Male | 133 (67.51%) |
| Female | 64 (32.49%) |
| Age | |
| Minimum | 20 |
| Maximum | 97 |
Figure 2: Clinical Characteristics and outcome of COVID 19 patients.
Clinical features of COVID-19 patients
Data shown in Table 2 indicated that the most frequent comorbidities among patients were diabetes at 62.44% followed by hypertension at 49.24%, cardiovascular diseases 17.77%, chronic kidney disease (CKD) at 8.12% while Hypothyroidism at 4.10% and respiratory disease at 2.53% (Figure 2). The baseline biochemical markers are presented in Table 3.
Table 2: Clinical characteristics of the study population.
| Comorbidities | N (%) |
|---|---|
| Diabetes | 123 (62.44%) |
| Hypertension | 97 (49.24%) |
| Cardiovascular disease | 35 (17.77 %) |
| Chronic kidney disease | 16 (8.12%) |
| Hypothyroidism | 8 (4.10%) |
| Respiratory disease | 5(2.53%) |
Table 3: Biochemical analysis of the study population.
| Parameters | Mean ± SD |
|---|---|
| 25(OH) D | 17.04 ± 11.18 |
| CRP | 17.15 ± 24.60 |
| HGB | 13.34 ± 2.18 |
| HCT | 44.48 ± 47.02 |
| Creatinine | 133 ± 186.53 |
| BUN | 11.18 ±13.44 |
| ALT | 53.22 ± 91.60 |
| AST | 59.83 ±101.33 |
The association between vitamin D levels and the severity of COVID-19
The association between vitamin D status and COVID-19 severity is presented in Table 4. The majority of the patients 144 (73.10%) had 25(OH)D<20 ng/ml while 31 (15.74%) patients had 25(OH)D ≥ 20 ng/ml and only 22 (11.17%) patients had 25(OH)D>30 ng/ml. No significant differences were found between COVID-19 patients admitted to general ward or intensive care unit (ICU) (p=0.67). Moreover, the main complications that patients developed during hospitalization were pneumonia 146 (74.11%), acute respiratory distress syndrome (ARDS) 30 (15.23%), acute kidney injury (AKI) 16 (8.12%) and septic shock 16 (18.12%) (Figure 2).
Table 4: Vitamin D according to severity of COVID-19.
| Clinical outcome | Serum vitamin D (ng/ml) | ||||
|---|---|---|---|---|---|
| - | Deficiency (<20 ng/ml) | Sufficient | Adequacy | - | |
| N=144 (73.10%) | (≥ 20 ng/ml) | (>30 ng/ml) | |||
| - | N=31 | N=22 | |||
| - | -15.74% | -11.17% | |||
| Ward admission | N (%) | P-value | |||
| General Ward | 79 | 16 | 14 | 109 (55.33%) | 0.67 |
| ICU | 65 | 15 | 8 | 88 (44.67%) | |
| Complications | |||||
| Pneumonia | 104 | 24 | 18 | 146 (74.11 %) | 0.96 |
| Acute respiratory distress syndrome | 21 | 5 | 4 | 30 (15.23%) | 0.85 |
| Acute kidney injury | 11 | 3 | 2 | 16 (8.12%) | 0.76 |
| Septic shock | 12 | 2 | 2 | 16 (8.12 %) | 1 |
| Mechanical ventilation | |||||
| Mechanical ventilation support | 40 | 7 | 3 | 50 (25%) | 0.34 |
| No mechanical ventilation support | 104 | 24 | 19 | 147(75%) | |
| Oxygen support | |||||
| No oxygen support | 30 | 5 | 3 | 38 (19.29%) | 0.49 |
| 1-5 L/min | 37 | 7 | 9 | 53 (26.90%) | |
| 6-10 L/min | 13 | 4 | 3 | 20(10.15%) | |
| 11-15 L/min | 19 | 7 | 4 | 30 (15.23%) | |
| >15 L/min or on MV | 45 | 8 | 3 | 56 (28.43%) | |
However, no statistically significant differences in any one of these complications were found among patients in the three vitamin D groups. Additionally, 50 (25%) patients required mechanical ventilation (MV) while 147 (75%) patients did not required mechanical ventilation (Figure 2). Also, oxygen support was provided for 53 (26.90%) patients with 1-5 L/min, 20 (10.15%) with 6-10 L/min, 30 (15.23%) patients with 11-15 L/min and 56 (28.43%) patients with >15 L/min or on MV, while 38 (19.29%) patients did not require oxygen support. No significant differences between MV or oxygen support were found among patients in the three vitamin D groups (p=0.34, p=49) respectively.
Clinical outcome of COVID-19 patients
The clinical outcome of COVID-19 patients is shown in Table 5 the mean of length stay on hospital was 8.65 ± 0.52 days. There was no correlation between serum 25(OH)D and length on hospital stay (r=0.06, P=0.41). Moreover, there is no association between serum 25(OH)D and CRP (r=-0.15. p=11).
Table 5: Clinical outcome of COVID-19 patients based on serum vitamin D.
| Clinical outcome | N (%) | Mean ± SD | R | P-value |
|---|---|---|---|---|
| Length of hospital stay | 197 (100%) | 8.65 ± 0.52 | 0.06 | 0.41 |
| CRP | 127 (64.5%) | 17.14 ± 2.18 | -0.15 | 0.11 |
| Clinical outcome | N (%) | Serum vitamin D (ng/ml) | F | P-value |
| Mean ± SD | ||||
| Discharge | 119 (60%) | 18.98 ± 1.12 | 3.81 | 0.02 |
| Hospitalize (transfer) | 56 (28 %) | 13.23 ± 0.97 | ||
| Deceased | 22 (11 %) | 16.20± 2.41 |
In addition, 119 (60%) patients were discharged with mean serum 25(OH)D 18.98 ± 1.12 and 22 (11%) patients deceased with mean serum 25(OH)D 16.20 ± 2.41 while 56 (28%) patients were transferred to another hospital to receive inpatient care with mean serum 25(OH)D 13.23 ± 0.97. There was a significant difference in outcome of COVID-19 patients (f=3.81, p=0.02) (Figure 3).
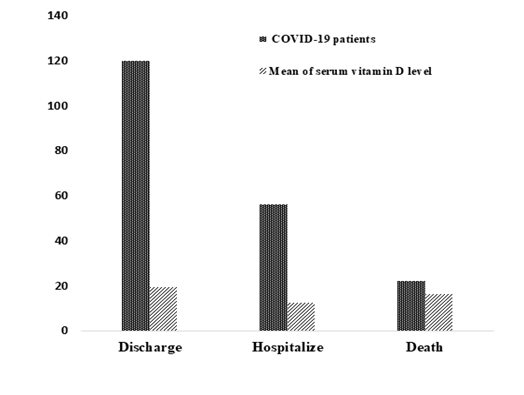
Figure 3: Number of COVID-19 patients with mean serum 25(OH)D.
Data shown in Table 6 indicated that the relationship of serum 25(OH)D level with clinical outcomes. There were no association between serum vitamin 25(OH)D and ICU admission, (Odd Ratio, OR 1.25 (95% confidence interval, CI, 0.41-3.88) P=0.70), MV support (Odd Ratio, OR 3.12 (95% confidence interval, CI 0.74- 13.21) p=0.12) and mortality (Odd Ratio, OR 2.39 (95% confidence interval, CI 0.31-18.11), p=0.40) in logistic regression after adjusted confounders.
Table 6: Multivariate logistic regression analysis for clinical outcome of COVID-19 patients.
| Multiple logistic regression | ||||||
|---|---|---|---|---|---|---|
| Clinical outcome | ICU admission | Mechanical ventilation support | Mortality | |||
| Covariance’s | 95%CI | P-value | 95%CI | P-value | 95%CI | P-value |
| Age | (0.97-1.00) | 0.25 | (0.96-1.00) | 0.16 | (0.92-0.98) | 0.002 |
| Gender | (0.96-3.46) | 0.07 | (0.62-2.75) | 0.49 | (0.31-2.45) | 0.79 |
| Diabetes | (0.71-2.54) | 0.37 | (1.16-5.67) | 0.02 | (1.05-15.76) | 0.04 |
| Hypertension | (0.37-1.39) | 0.32 | (0.36-1.61) | 0.48 | (0.16-1.36) | 0.16 |
| CKD | (0.49-4.08) | 0.53 | (0.37-3.84) | 0.77 | (0.63-1.00) | 0.18 |
| Adjusted aOR vit.D | 1.25 | 3.12 | 2.39 | |||
| P-value | 0.7 | 0.12 | 0.4 | |||
| 95%CI | (0.41-3.88) | (0.74-13.21) | (0.31-18.11) | |||
| Note: *Significant level p<0.05 OR=Odds Ratio CI=Confidence Interval a Logistic regression model contain vitamin D level as a continues variable adjusted by age, gender, diabetes, hypertension and chronic kidney disease (CKD). | ||||||
However, in a logistic regression model containing serum 25(OH) D, diabetes was associated significantly with MV support with a 95% confidence interval, CI 1.16-5.67 P=0.02. Age and diabetes were associated significantly with mortality with a 95% confidence interval, (CI 0.92-0.98, P=0.002) and 95% confidence interval, (CI 1.05-15.76, P=0.04), respectively.
To investigate the hypothesis that vitamin D status is associated with COVID-19 severity. The objective of this study was to examine the association of vitamin D levels with admission to the ICU, length of hospital stay and clinical outcome of 197 hospitalized COVID-19 patients in the Western region of KSA. The most important clinically relevant finding in the current study indicated that vitamin D deficiency was highly prevalent among the study population at 73.10%. This result is in line with Alguwaihes et al. performed a single center retrospective study involving 439 COVID-19 patients in the Riyadh region [26]. They found that 74.7% of patients had a vitamin D deficiency, which is considered as one of the predictive factors of mortality [26]. This finding was expected as vitamin D deficiency is common in Saudi Arabia [27].
According to our analysis, the hospitalized and deceased patients had lower mean serum vitamin D compared with discharged patients. Low levels of vitamin D have been associated with comorbidities specifically in elderly population [28-30]. This might explains the current observations of this study as it has been recently observed the more severe COVID-19 cases were common among elderly with comorbidities [31]. The current study indicates that diabetes and age are the most significant predictors of the association between serum 25(OH)D and mortality rate among COVID-19 patients. In contrast, Alguwaihes et al. demonstrated in a retrospective study conducted in Riyadh, KSA that diabetes not associate with mortality rate after control of covariant among 439 COVID-19 [26]. However, it is noteworthy in this study that vitamin D deficiency was not associated with mortality after adjustment of age, gender and comorbidities such as type 2 DM, hypertension and CKD. This agrees with the results of a study conducted in the USA, involving 93 COVID-19 patients. No association between vitamin D status and the mortality rate was found [32]. Moreover, this study did not find any association between serum vitamin D levels and adverse outcome of COVID-19 patients as well as risk for ICU admission and MV support, these findings were confirmed in multi logistic regression after adjustment of covariates. The Szeto et al. results are aligned with the data from a retrospective case-control study that included 216 COVID-19 patients and 197 population-based control patients [32]. They reported no association between serum vitamin D level and severity of COVID-19 symptoms, including ICU admission and intubation [33].
The present study's findings do not support the previous research which have suggested that vitamin deficiency was common among critical ill patients and associated with worse outcome [34,35]. This inconsistency may be due to the difference in ethnic background, age group and the modest sample size. In the other hand, Radujkovic et al. conducted a retrospective observational study among 185 COVID-19 patients in Germany, they demonstrated that patients with vitamin D deficiency had 6 fold higher risk for developing a more sever course of the disease including requirement of invasive mechanical ventilation (IMV) and 15 fold higher risk for death [36].
Furthermore, no association was detected in the current study between the serum vitamin D level and length of hospital stay in a multi linear regression analysis. This result was also reported by Orchard et al. who performed a cohort observational study among 165 elderly patients and found that the serum vitamin D level was not associated with the numbers of days of hospitalization [37]. In contrast, Demir et al. demonstrated that COVID-19 patients with serum vitamin D>30 ng/ml had shorter hospital stay in a retrospective cohort study including 227 patients [38]. Possible explanations for this result may be the small sample size, the confounder’s factors were not controlled, and relying on prehospitalization serum vitamin D values measured within six months before the diagnosis of COVID-19, rather than measuring during hospitalization.
These various studies indicate that vitamin D deficiency alone cannot fully explain the severity of COVID-19. Growing body of evidence supports the link between vitamin D supplementation and COVID-19 severity. A pilot randomized control trial involving 76 COVID-19 patients in Spain showed that 98% of the treated patients with calcifediol did not require ICU admission and did not die, while 50% of the untreated patients were admitted to the ICU and two died [24]. In addition, Annweiler et al. conducted a quasi-experimental study among 77 frail elderly COVID-19 patients and found that COVID-19 patients on regular vitamin D supplementation over the preceding year of 50.000 IU/month or 80,000 IU or 100000 IU every 2-3 months had lower severity of COVID-19 (Odds Ratio (OR)=0.08 (95% CI): 0.01; 0.81), (p=0.033) [39].
The absence of significant risk in COVID-19 severity among patients with hypovitaminosis D observed in the present study does not supersede the fact that vitamin D have an important role in supporting immune system [14,40]. Thus, it is important to reach sufficient levels to avoid symptoms of deficiency and comorbidities.
Strength and limitation
To the best of our knowledge this retrospective study was the first multicenter study included three hospitals in the western region of SA to assess the association between serum vitamin D level and severity of COVID-19. The categorization of vitamin D was based on local recommendations to diagnosis and treatment vitamin of vitamin D deficiency. Multivariate regression was used in order to control the effect of possible confounders.
This study had several potential limitations as it was a retrospective study and selection bias may be concerned. The sample size was small as power calculation estimated at 80%. Some confounder’s factors such as dietary assessment, physical activity, ethnicity, smoking status, BMI and socioeconomic status weren’t considered for patients. Vitamin D was measured for most patients during hospitalization after COVID-19 had advanced and developed them to acute phase rather than measure it upon first admission. The critical illness affected vitamin D binding protein which affects the vitamin D bioavailability [41].
Some patients were undergone to vitamin D supplementation during hospitalization and did not take into consideration. Inflammatory markers such as CRP and ferritin weren’t available for most patients. Despite these limitations, the finding of this study added value to literature as the evidence on COVID-19 patients within Middle East are limited with mix result.
In conclusion, the prevalence of vitamin D deficiency is high among COVID-19 patients in the Western region of SA. The lack of association was detected in the present study between serum vitamin D and severity of COVD-19 including ICU admission, intubation, mortality and days of hospitalization. Further large randomized control tail studies covering multiple institutions are needed to determine the therapeutic effect of vitamin D supplementation on COVID-19 severity.
The author thank Dr. Hussain S. Baubad consultant of internal medicine in Complex King Faisal Hospital in Taif for assessing the vitamin D level of COVID-19 patients. Special thanks for all laboratory medical staffs in King Faisal Hospital and Al Noor Specialist Hospital in Makkah for all efforts. Sincere thanks and appreciation to his excellency Prof. Dr. Hameda El-Sayed Ahmed El-Sayed for the preparation of the article for publication and communication with the scientific journal for the publication.
Authors have no conflicts of interest.
Citation: Alquthami FR, Qadhi AH, Mustafa RA, Ghafouri KJ (2021) The Impact of Vitamin D Status on COVID-19 Severity among Hospitalized Patients in the Western Region of Saudi Arabia, A Retrospective Cross-Section Study. J Nutr Food Sci. 11:826.
Received: 12-Nov-2021 Accepted: 26-Nov-2021 Published: 03-Dec-2021 , DOI: 10.35248/2155-9600.21.s9.1000828
Copyright: �© 2021 Alquthami FR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.