PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2019) Volume 10, Issue 1
The Effects of Switching from Fluticasone/Salmeterol Aerosol to Fluticasone/Formoterol Aerosol in Patients with Severe Asthma
Soichiro Hanada1, Ken Shirahase1, Hirochiyo Sawaguchi1, Masato Muraki1* and Yuji Tohda22Department of Respiratory Medicine and Allergology, Kindai University Hospital, Japan
Received: 02-Mar-2019 Published: 18-Mar-2019
Abstract
Objective: Two pressurized Metered Dose Inhalers (pMDIs) containing a combination of a corticosteroid and long acting β2 agonist: Fluticasone propionate/Salmeterol Combination (FSC), and Fluticasone propionate/Formoterol Combination (FFC), are currently available in Japan. In order to examine the usefulness of the FFC pMDI, we sought to investigate the efficacy, adverse events, and handling after switching to the FFC pMDI from the FSC in patients with asthma.
Methods: Fifty-six outpatients who were using the FSC pMDI (250/50 μg) twice daily were enrolled in the study. The following items were evaluated before and after the use of the FFC pMDI (250/10 μg) twice daily for 8 weeks: Asthma Control Test (ACT) Questionnaire; Asthma Health Questionnaire (AHQ)-33-Japan; spirometry; and forced
oscillation technique. On the final day of the study, the original questionnaire was administered for a subjective evaluation between the FSC pMDI and FFC pMDI.
Results: We found improvements in ACT (22.54 to 22.98, p=0.0076) and AHQ-33 Japan (16.27 to 14.23, p=0.0162) scores after FFC use. In addition, inspiratory capacity (p <0.0001) and forced expiratory volume in 1 second (p=0.0122) significantly increased. Further, 51.8% of patients preferred the FFC, while 12.5% preferred the FSC.
Conclusion: There was a subjective and objective improvement in patients after switching to FFC pMDI from FSC pMDI. These findings indicate that FFC is more useful for patients with asthma. Further studies are needed to confirm the results of this study.
Keywords
Asthma; Fluticasone propionate; Salmeterol; Formoterol; Metered dose inhalers; Asthma control test; Asthma health questionnaire
Introduction
Asthma is an inflammatory disease of the airway. The first choice of medication for asthma management is Inhaled Corticosteroids (ICS). However, if asthma control is insufficient with ICS monotherapy, a combination of ICS and Long Acting β2 Agonist (LABA) is recommended [1,2]. Currently, five types of devices for four ICS/LABA combinations are available in Japan. The fluticasone propionate/salmeterol combination and fluticasone propionate/formoterol combination treatments are available as pressurized metered dose inhaler devices: FSC pMDI, Adoair® 125 Aerosol, marketed in April 2010; and FFC pMDI, Flutiform® 125 Aerosol, marketed in December 2014 in Japan.
In a previous study, we found that patients with asthma preferred pMDI to dry powder inhaler, if the ICS/LABA combinations are similar [3]. However, differences in the effects of medications among individuals can be associated with differences in drug efficacy or inhalation technique [4,5]. For example, formoterol has a faster effect than salmeterol [6]. In addition, the aerosol transfer velocity in FFC pMDI is slower than FSC pMDI [7], suggesting that the inhalation efficiency of FFC pMDI is superior. However, no comparative studies have assessed these medications, and there may also be individual differences in patient preference.
In the present study, we sought to investigate the efficacy, adverse events, and handling of patients with asthma who switched from FSC pMDI to FFC pMDI.
Methods
Outpatients with severe asthma who had been attending the Department of Respiratory Medicine and Allergology at Kindai University Nara Hospital (Ikoma, Japan) were recruited. Inclusion criteria were: requiring Step 4 treatment [1]; and receiving treatment with the FSC pMDI, Adoair® 125 Aerosol, (2 puffs, 250/50 μg, twice daily). Exclusion criteria were: <20 years of age; inability to inhale unassisted; inability to perform spirometry tests; pregnancy; severe comorbidities affecting Quality of Life (QoL), such as malignancy, cardiac failure, renal failure, severe liver dysfunction or respiratory failure; and current smoker status. Informed consent was obtained from all participants.
This study was performed as prospective open-labelled clinical observational study. Asthma patients were switched from the FSC pMDI to the FFC pMDI, Flutiform® 125 Aerosol (2 puffs, 250/10 μg, twice daily), for 8 weeks. Changes in concomitant medications were prohibited in the 8 weeks before the examination and throughout the study period.
Participants ’ background characteristics were recorded. In addition, we assessed participants using the asthma control test Questionnaire [1,8,9]; the asthma health questionnaire-33-Japan [2,10-12]; physical findings; spirometry using a Chestac-33 (Chest M.I., Tokyo, Japan); and respiratory system resistance and reactance as determined via the Forced Oscillation Technique (FOT) using MostGraph-01 (Chest M.I., Tokyo, Japan) [13,14]. Participants were assessed before and after the use of FFC pMDI. On the final day of the study, participants completed a questionnaire for the subjective assessment of handling, effects, side effects, overall evaluation, and selection of the treatments (Supplemental Table 1).
| N | 56 | Spirometry | ||
|---|---|---|---|---|
| Male:Female | 29:27 | FVC (L) | 2.83 ± 1.02 | |
| Age (years old) | 68.1 ± 14.5 (25-90) | %FVC (%) | 95.3 ± 19.9 | |
| Pediatric asthma | 7 (12.7%) | FEV1 (L) | 1.91 ± 0.72 | |
| Disease duration (y) | 9.8 ± 10.8 | %FEV1 (%) | 81.2 ± 22.2 | |
| Allergic rhinitis | 31 (55.4%) | FEV1/FVC (%) | 68.3 ± 13.0 | |
| Smoking history | Never | 30 | Post BD FEV1 (L) | 2.01 ± 0.81 |
| Former | 26 | % Post BD FEV1 (%) | 85.1 ± 23.3 | |
| Height (cm) | 158.7 ± 9.5 | Concomitant medications (Controller except for FSC pMDI) | ||
| Body weight (kg) | 60.2 ± 11.4 | |||
| BMI (kg/cm2) | 23.8 ± 3.3 | LTRA | 30 | |
| Theophylline | 7 | |||
| Tiotropium | 4 | |||
| Mucolytic | 6 | |||
| PSL | 1 | |||
Table 1: Patient characteristics. BMI: Body Mass Index; FVC: Forced Vital Capacity; FEV1: Forced Expiratory Volume in 1 second; BD: Bronchodilator; LTRA: Leukotriene Receptor Antagonists; PSL: Prednisolone.
All examinations were performed between 09:00 and 11:00, which was 2-4 hours after the patients had administered their normal morning medications, including FSC. The examination start times between before and after the study were also matched. The use of any Short-Acting β-Agonist (SABA) within 12 hours of the visit was prohibited. Any postponement of the evaluation appointment was permitted for up to 4 days.
The study protocol was approved by the Institutional Review Board at Kindai University Nara Hospital, and was implemented in compliance with the Declaration of Helsinki.
Statistical analysis
Data are presented as mean ± standard deviation. Statistical differences were assessed using paired t-tests for before and after treatment effects. Statistical analyses were performed using JMP® version 10.0.2 (SAS Institute Japan, Tokyo, Japan). p <0.05 was considered statistically significant.
Results
Fifty-six subjects were initially enrolled in the study. Their characteristics are shown in Table 1.
No participants had severe infectious diseases and dropped out during the study; however, the FOT was not performed or was immeasurable in 7 individuals.
The average ACT total score significantly improved from 22.54 to 22.98 after FFC pMDI treatment (p=0.0076). In addition, each domain, except for the use of rescue medication, showed significant improvements (Table 2).
| FSC pMDI | FFC pMDI | p-value | ||||
|---|---|---|---|---|---|---|
| ACT | ||||||
| Q1 | Influence at work or at home? | 4.70 ± 0.66 | 4.80 ± 0.44 | 0.0163 | ||
| Q2 | Shortness of breath | 4.36 ± 1.05 | 4.45 ± 1.08 | 0.029 | ||
| Q3 | Wake up at night or early morning? | 4.46 ± 1.04 | 4.64 ± 0.90 | 0.0085 | ||
| Q4 | Use of rescue medication | 4.86 ± 0.44 | 4.89 ± 0.37 | 0.0796 | ||
| Q5 | Asthma control | 4.13 ± 0.88 | 4.25 ± 0.79 | 0.0352 | ||
| Total | 22.54 ± 3.00 | 22.98 ± 2.75 | 0.0076 | |||
| AHQ-33 Japan | ||||||
| AS | Asthmatic Symptom | 4.84 ± 4.91 | 4.21 ± 4.03 | 0.0598 | ||
| FWS | Factors with Worsened Symptoms | 3.98 ± 4.85 | 3.52 ± 4.23 | 0.0096 | ||
| Em | Emotion | 4.13 ± 4.77 | 3.45 ± 3.87 | 0.014 | ||
| DA | Daily Activity | 1.52 ± 2.32 | 1.43 ± 2.27 | 0.029 | ||
| SA | Social Activity | 1.45 ± 2.34 | 1.25 ± 2.06 | 0.0236 | ||
| Ec | Economics | 0.36 ± 0.75 | 0.38 ± 0.82 | 0.8392 | ||
| Total | 16.27 ± 16.99 | 14.23 ± 13.83 | 0.0162 | |||
| Face scale | 1.20 ± 0.86 | 0.95 ± 0.77 | 0.0003 | |||
Table 2: Quality of life after treatment with FSC or FFC pMDI. QoL: Quality of Life; ACT: Asthma Control Test; AHQ: Asthma Health Questionnaire; FSC: Fluticasone propionate/Salmeterol Combination; FFC: Fluticasone propionate/Formoterol Combination; pMDI: pressurized Metered Dose Inhaler.
Inspiratory Capacity (IC) and Forced Expiratory Volume in one second (FEV1) were significantly improved after 8 weeks of FFC treatment (IC, p <0.0001, FEV1, p=0.0122; Table 3).
However, there were no significant differences in FOT parameters at average phase. In addition, there were no significant differences in FOT parameters at expiratory, inspiratory, and expiratory minus inspiratory phases (Supplemental Table 2).
| FSC pMDI | FFC pMDI | p-value | |
|---|---|---|---|
| Spirometry | |||
| IC (L) | 2.01 ± 0.68 | 2.12 ± 0.71 | <0.0001 |
| FVC (L) | 2.81 ± 1.02 | 2.81 ± 0.99 | 0.4085 |
| FEV1 (L) | 1.86 ± 0.70 | 1.90 ± 0.69 | 0.0122 |
| PF (L/s) | 5.56 ± 2.05 | 5.72 ± 2.06 | 0.0587 |
| V50 (L/s) | 1.82 ± 1.22 | 1.78 ± 1.03 | 0.6177 |
| V25 (L/s) | 0.46 ± 0.31 | 0.47 ± 0.32 | 0.3094 |
| V50/V25 | 4.12 ± 1.48 | 4.12 ± 1.31 | 0.5105 |
| MostGraph (Average) (n=49) | |||
| R5 | 3.69 ± 1.68 | 3.54 ± 1.51 | 0.1749 |
| R20 | 3.04 ± 1.20 | 2.91 ± 1.08 | 0.0928 |
| R5-R20 | 0.65 ± 0.57 | 0.63 ± 0.58 | 0.4164 |
| X5 | -1.28 ± 1.49 | -1.10 ± 1.30 | 0.8777 |
| Fres | 11.03 ± 4.98 | 10.62 ± 5.05 | 0.1735 |
| ALX | 8.52 ± 13.18 | 7.27 ± 11.92 | 0.1856 |
Table 3: Spirometry and Forced Oscillation Technique (MostGraph®) after treatment with FSC or FFC pMDI. FSC: Fluticasone propionate/Salmeterol Combination; FFC: Fluticasone propionate/Formeterol Combination; pMDI: pressurized Metered Dose Inhaler; IC: Inspiratory Capacity; FVC: Forced Vital Capacity; FEV1: Forced Expiratory Volume in the first second; PF: Peak expiratory Flow; V50: forced expiratory flow at 50% vital capacity; V25: forced expiratory flow at 25% vital capacity; R5: Resistance at 5 Hz; R20: Resistance at 20 Hz; X5: reactance at 5 Hz; Fres: Frequency of resonance; ALX: Low-frequency reactance Area.
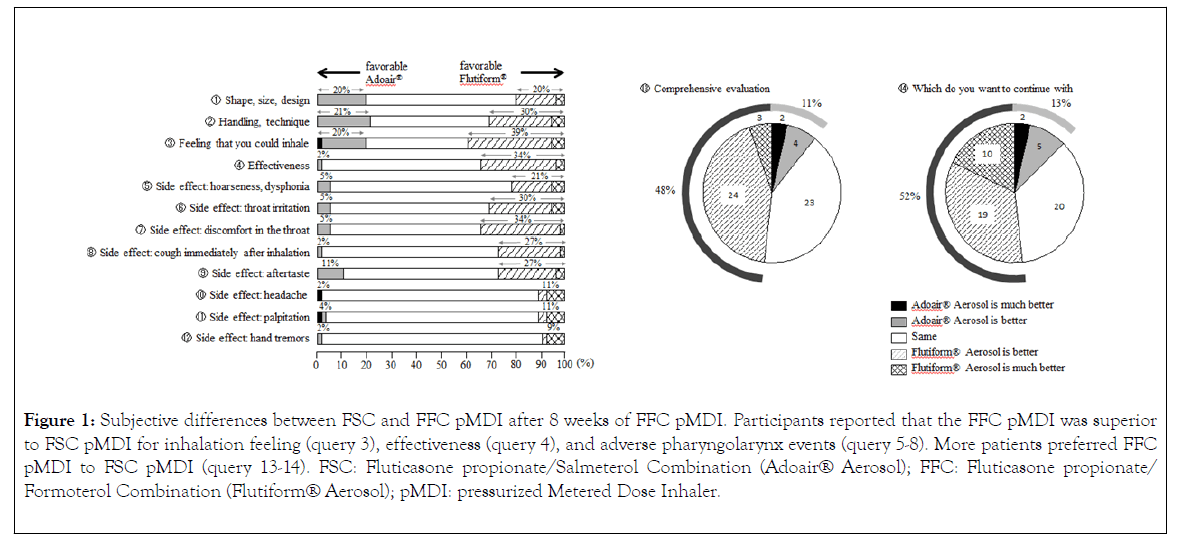
At the end of the study, participants answered a questionnaire for a subjective comparison of the FSC pMDI and FFC pMDI (Figure 1). FFC pMDI was superior to FSC pMDI in the following items: 3) “ feeling that you could inhale ” and 4) “effectiveness”. FFC pMDI was also superior to FSC pMDI with regard to adverse events of the pharyngolarynx, such as 5) “ hoarseness ” , 6) “ throat irritation ” , 7) “ discomfort in the throat, ” and 8) “ cough immediately after inhalation. ” Furthermore, more participants preferred FFC pMDI to FSC pMDI.
Figure 1: Subjective differences between FSC and FFC pMDI after 8 weeks of FFC pMDI. Participants reported that the FFC pMDI was superior to FSC pMDI for inhalation feeling (query 3), effectiveness (query 4), and adverse pharyngolarynx events (query 5-8). More patients preferred FFC pMDI to FSC pMDI (query 13-14). FSC: Fluticasone propionate/Salmeterol Combination (Adoair® Aerosol); FFC: Fluticasone propionate/ Formoterol Combination (Flutiform® Aerosol); pMDI: pressurized Metered Dose Inhaler.
However, the free comments revealed that there were 13 subjects who found it difficult to understand the residual quantity of the FFC pMDI, one who found that more power was required for puffing in the FFC pMDI, and one who commented on a worse aftertaste in the FFC pMDI.
Discussion and Conclusion
In this study, we sought to compare the effect of changing from FSC pMDI to FFC pMDI treatment in patients with asthma. The first ICS/LABA combination available in Japan as a pMDI was the FSC pMDI. The FFC pMDI (120 inhalation device) has been available since 2014 in Japan, and studies have shown its efficacy for asthma [15,16]. We found improvements in QoL and pulmonary functions in participants who switched to FFC pMDI in the present study. There may be some bias due to the study design and an open-labelled trial; however, we found that FFC pMDI was more useful than FSC pMDI. In line with this, a previous study reported that fluticasone/formoterol (100/10 μg or 250/10 μg twice daily) was equally as effective as fluticasone/ salmeterol (100/50 μg or 250/50 μg twice daily), and has a more rapid onset of action. This reflects the faster bronchodilatory effects of formoterol when compared with salmeterol [15].
The ACT is a simple QoL questionnaire that is used worldwide [1,8,9]. The AHQ-33-Japan is a validated QoL questionnaire recommended by Japanese guidelines [2] that is widely used in Japan [10-12]. In the present study, there was no difference in the frequency of rescue medication, as measured by the ACT; however, we found that QoL was improved as measured by all other items. In addition, the AHQ-33-Japan showed no significant difference in asthmatic symptom and economics; however, there was a significant improvement in all other domains, including face scale. Thus, the participants’ QoL was improved by switching to FFC pMDI.
The US Food and Drug Administration recommend the addition of formoterol or salmeterol as a combination inhaler [17]. We have shown that FFC pMDI showed improved spirometry compared with FSC pMDI in our study. This is most likely to be due to differences in responsiveness between the LABAs, formoterol, and salmeterol, in each medication because the dosage of corticosteroid (fluticasone propionate) did not change. Formoterol has a more rapid onset than salmeterol [6]; with 50 μg salmeterol estimated to be equipotent to 9 μg formoterol [6]. Due to the difference in acute onset, we measured spirometry and FOT at a later stage so that the acute post-inhalation effect had plateaued. Interestingly, inhaled formoterol (12 μg twice daily) or salmeterol (50 μg twice daily) shows similar effects on Peak Expiratory Flow (PEF), mean daytime symptom score, or night time symptom score in patients with severe asthma who had been treated with ICS [18]. However, a previous study has shown that formoterol has a stronger relaxant effect on methacholine-induced contraction in animal airway smooth muscle [19]. In addition, formoterol is more effective than salmeterol in combination with corticosteroids in the treatment of respiratory disease under conditions of high oxidative stress [20].
An alternative hypothesis for our results is that device differences result in differences to the arrival and deposition of drug particles into the airway. Compared with DPI, pMDI has a smaller drug aerosol particle diameter and higher aerosolization rate, which results in more efficient travel to the peripheral airway [21]. Further, it has been reported that a slower aerosol transfer velocity is more effective [7], and that aerosol particles of 1-5 μm in size can travel from the periphery to the central airway [22]. The percentage of aerosols use this particle size are approximately 93% and 85% for FFC pMDI and FSC pMDI, respectively. In addition, the aerosolization percentages are 95.0% and 84.6% for FFC pMDI and FSC pMDI, respectively [7]. In addition, the aerosol transfer velocity of FFC pMDI was slower than FSC pMDI. This is a key feature of the inhalation efficiency of pMDI: the aerosol transfer velocities at 80 mm and 100 mm from the nozzle are 6.04 m/sec and 5.14 m/sec for FFC pMDI and 8.69 m/sec and 7.13 m/sec for FSC pMDI, respectively [7]. This indicates that it is easier for patients to synchronize and the inhalation efficiency is higher for FFC pMDI than FSC pMDI [7]. Furthermore, the plume of FFC pMDI is warmer and less forceful than that of FSC pMDI, which creates a longer plume duration and slower maximal velocity [23]. These differences may produce differences in the deposition of each drug on pharyngolarynx and contribute to local adverse events of the pharyngolarynx, such as throat irritation.
A limitation of the present study is that it comprised a realworld switching study, rather than a randomized controlled study or placebo-controlled study.
Our study indicates that the use of FFC improved lung function and QoL. We hypothesize that the rapid onset of formoterol effects and the use of a superior device were associated with the increased preference in this study. A previous comparative study of the same dose of FSC pMDI and FSC dry powder inhalers (Diskus®) in patients moderate to severe asthmatic found a preference for the pMDI device [3]. Notably, patients ’ preferences may lead to changes in adherence and effects of medications [24]. Further, inhalation devices can cause confusion for the patient, because the formulations in these DPI devices are diverse. Conversely, although there are minor differences in pMDI, the inhalation techniques are largely similar among pMDI devices, including SABA. Taken together, FFC pMDI may be the first choice of ICS/LABA treatment for patients with asthma
REFERENCES
- Global Initiative for Asthma (GINA). 2018 GINA Report, Global Strategy for Asthma Management and Prevention. 2018.
- Ichinose M, Sugiura H, Nagase H, Yamaguchi M, Inoue H, Sagara H, et al. Japanese guidelines for adult asthma 2017. Allergol Int. 2017;66(2): 163-189.
- Muraki M, Gose K, Hanada S, Sawaguchi H, Tohda Y. Which inhaled corticosteroid and long-acting ß-agonist combination is better in patients with moderate-to-severe asthma, a dry powder inhaler or a pressurized metered-dose inhaler? Drug Deliv. 2017;24(1): 1395-1400.
- Weers J, Clark A. The Impact of Inspiratory Flow Rate on Drug Delivery to the Lungs with Dry Powder Inhalers. Pharm Res. 2017;34(3): 507-528.
- Bonds RS, Asawa A, Ghazi AI. Misuse of medical devices: a persistent problem in self-management of asthma and allergic disease. Ann Allergy Asthma Immunol. 2015;114(1): 74-76.
- Palmqvist M, Persson G, Lazer L, Rosenborg J, Larsson P, J Lotvall. Inhaled dry-powder formoterol and salmeterol in asthmatic patients: onset of action, duration of effect and potency. Eur Respir J. 1997;10(11): 2484-2489.
- Tamura G. Performance of metered-dose inhaler of combination agents for bronchial asthma. Resp Res. 2013;32: 1075-1080.
- Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124(4): 719-723.
- Thomas M, Kay S, Pike J, Williams A, Rosenzweig JR, Hillyer EV, et al. The Asthma Control Test (ACT) as a predictor of GINA guideline-defined asthma control: analysis of a multinational cross-sectional survey. Prim Care Respir J. 2009;18: 41-49.
- Arioka H, Kobayashi K, Kudo K, Kabe J. Validation study of a disease-specific module, the Asthma Health Questionnaire (AHQ) using Japanese adult asthmatic patients. Allergol Int. 2005;54(3): 473-482.
- Muraki M, Ichihashi H, Haraguchi R, Iwanaga T, Kubo H, Tohda Y. Comparison of the Asthma Health Questionnaire-33-Japan and the short-form 36-item health survey for measuring quality of life in Japanese patients with asthma. Allergol Int. 2008;57(4): 339-346.
- Tohda Y, Iwanaga T, Sano H, Kume H, Hirata K, Ohkura N, et al. Improved quality of life in asthma patients under long-term therapy: Assessed by AHQ-Japan. Int J Clin Pract. 2017;71(1).
- Abe Y, Shibata Y, Igarashi A, Inoue S, Sato K, Sato M, et al. Reference values of Most Graph measures for middle-aged and elderly Japanese individuals who participated in annual health checkups. Respir Investig. 2016;4(3): 148-155.
- Shirai T, Kurosawa H. Clinical application of the forced oscillation technique. Intern Med. 2016;55(6): 559-566.
- Bodzenta-Lukaszyk A, Dymek A, McAulay K, Mansikka H. Fluticasone/formoterol combination therapy is as effective as fluticasone/salmeterol in the treatment of asthma, but has a more rapid onset of action: an open-label, randomized study. BMC Pulm Med. 2011;11: 28.
- Prosser TR, Bollmeier SG. Fluticasone-formoterol: a systematic review of its potential role in the treatment of asthma. Ther Clin Risk Manag. 2015;11: 889-899.
- Cates CJ, Wieland LS, Oleszczuk M, Kew KM. Safety of regular formoterol or salmeterol in adults with asthma: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;6(2): CD010314.
- Nightingale JA, Rogers DF, Barnes PJ. Comparison of the effects of salmeterol and formoterol in patients with severe asthma. Chest. 2002;121(5): 1401-1406.
- Kume H, Fukunaga K, Oguma T. Research and development of bronchodilators for asthma and COPD with a focus on G protein/KCa channel linkage and ß2-adrenergic intrinsic efficacy. Pharmacol Ther. 2015;56: 75-89.
- Rossios C, To Y, Osoata G, Ito M, Barnes PJ, Ito K. Corticosteroid insensitivity is reversed by formoterol via phosphoinositide-3-kinase inhibition. Br J Pharmacol. 2012;167(4): 775-786.
- Goldin JG, Tashkin DP, Kleerup EC, Greaser LE, Haywood UM, Sayre JW, et al. Comparative effects of hydrofluoroalkane and chlorofluorocarbon beclomethasone dipropionate inhalation on small airways: assessment with functional helical thin-section computed tomography. J Allergy Clin Immunol. 1999;104(6): S258-267.
- Consensus Conference on Aerosol Delivery. Aerosol consensus statement. Chest. 1991;100(4): 1106–1109.
- Johal B, Murphy S, Tuohy J, Marshall J. Plume Characteristics of Two HFA-Driven Inhaled Corticosteroid/Long-Acting Beta2-Agonist Combination Pressurized Metered-Dose Inhalers. Adv Ther. 2015;2(6): 567-579.
- Hanada S, Wada S, Ohno T, Sawaguchi H, Muraki M, Tohda Y. Questionnaire on switching from the tiotropium HandiHaler to the Respimat inhaler in patients with chronic obstructive pulmonary disease: changes in handling and preferences immediately and several years after the switch. Int J Chron Obstruct Pulmon Dis. 2015;10(1): 69-77.
Citation: Hanada S, Shirahase K, Sawaguchi H, Muraki M, Tohda Y (2019) The Effects of Switching from Fluticasone/Salmeterol Aerosol to Fluticasone/Formoterol Aerosol in Patients with Severe Asthma. J Allergy Ther 10: 288. doi:10.4172/2155-6121.1000288
Copyright: © 2019 Hanada S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.