PMC/PubMed Indexed Articles
Indexed In
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Scimago
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 10, Issue 12
Survival Rate and Early Development of Giant Gourami Padang Strain Osphronemus gouramy (Perciformes: Osphronemidae)
Livia Rossila Tanjung*Received: 21-Oct-2019 Published: 16-Dec-2019
Abstract
Aquaculture of gourami in Indonesia is still carried out traditionally with a simple touch of technology that makes the mortality rate of larvae is still very high. This study aimed to determine the survival rate and development of giant gourami Padang strain larvae Osphronemus gouramy reared in a traditional way and to gain insight into the cause of mass death during the larval period. The larvae were obtained from six pairs of broodstocks that consisted of three categories, which is the grey broodstocks that spawned the previous month, the grey broodstocks that did not spawn the previous month, and the pink broodstocks that did not spawn the previous month, and were named after the body colour of their respective parents. The study was conducted in duplicate and the larvae were sampled for photography every day and the larvae development was described from Day 1 to Day 10. The eggs began to hatch on Day 2, and all eggs hatched on Day 3 that marked the end of the embryonic stage. Pale melanophores became more intense on Day 6 and the yolk sac was still visible on Day 10. The mass deaths during embryogenesis are thought to be influenced by factors such as broodstock quality, while the ones occurring in the late larval period are more likely related to water quality and stocking density. Ammonia excretion subsequently oxidized into more toxic nitrite was the only possible pollutant to be present in the rearing water. In the Grey II.1 and Grey II.2 basins where the mass death occurred on Day 10, the mean of survival rates declined significantly from 83.45% on Day 9 to 32.15% on Day 10. Thus, this study confirmed that the basins with larvae density of above 30 individuals per litre can support the larvae rearing for eight days and on Day 9 the larvae must be transferred to another tank. The success of larval production depends not only on the welfare and the feed quality of the broodstocks but also on the optimal environment in larval rearing. In addition, the nomenclature for the larval stage of gourami was also discussed and determined.
Keywords
Development; Giant gourami Padang strain; Larvae; Osphronemus gouramy; Survival rate
Introduction
Giant gourami Osphronemus gouramy Lacépède, 1802 belonging to the family Osphronemidae and suborder Anabantoidei [1], is one of the most common freshwater omnivorous fishes in Southeast Asia [2]. The fish is thought to be native to Indonesian fresh waters which initially spread in Sumatra based on the discovery of fossils of gourami collected from probable Eocene deposits of the Sangkarewang Formation in the Ombilin Basin of Sumatra [3]. In nature, the fish inhabit rivers, lakes, swamps, and ponds, but they prefer shallow waters with aquatic plants. Occasionally, these fish surface to breathe directly from the air [2].
Gourami is one of the leading commodities in freshwater aquaculture due to its high economic value and continuously increasing market demand thanks to its flavour and flesh quality. This species can reach a length of 70 cm [1], but the common consumption size is 0.5–1.0 kg or about 40–50 cm long. The growth rate of gourami is slower than that of common carp, catfish, or tilapia, but its selling price is about double the price of common carp, catfish, or tilapia [4].
Depending on the geographical regions, there are different strains of gourami that have been cultivated in Indonesia. In Java, the strains commonly cultured are gourami Soang, Jepun, Bastar, Paris, and Blusafir. In Sumatra, the fish growers prefer to culture local gourami strains such as gourami Padang, Sago, and Tambago because the strains originated from Java do not grow well in the Sumatran environment, while in Borneo the local strain Mandiangin that is chosen for aquaculture. So far, these different strains were named as such following the names given by the fish growers who distinguish strains based on morphological characteristics such as shapes of the mouth or forehead, length of pelvic fins, body colours, and the number of eggs produced.
There are two types of gourami Padang: the pink and the grey gourami, which differ one from another by body colour and the number of eggs. The pink gourami Padang is of interest since it was shown to have a natural resistance against Aeromonas infection [5], while the resistance to Aeromonas of the grey fish is still unknown. Thus, due to its natural resistance, the pink gourami has the potential to be developed for large scale aquaculture. However, a pink female only produces around 2,000 eggs compared to a grey female that usually spawns about 4,000 eggs during a spawning period.
The larval stage is a critical period in the life of gourami, like any other fish species. During this period, internal organs and tissue systems, particularly the digestive system start to establish [6-8]. The mortality is the highest during the larval stage in the gourami culture. The traditional gourami growers often experience up to 50% mortality during this period.
This study was intended to determine the survival rate of giant gourami Padang strain larvae reared traditionally and to obtain the morphological events related to gourami embryogenesis and its larval ontogenesis since this information are lacking for the Padang strain of gourami. Another purpose of the study was to find the optimum period for rearing the larvae in the same basin and to gain insight into the cause of mass death during the larval period. All of the above information regarding the early stages of gourami development should be useful in overcoming the problem of mass death during the culture of gourami larvae.
Materials and Methods
Broodstock maintenance and egg collection
This study was performed at a gourami farm in Padang, West Sumatra, for 10 days from November 8th, 2016. The two types of gourami Padang strain were utilized in this study: the grey and the pink gourami. Each pair of broodstocks formed of one male and one female was maintained in a compartment measuring 2 x 2 x 0.6 m3 inside a pond (Figure 1) to spawn naturally. The spawning compartments were perfectly separated with each other using nets so that the fish cannot be mixed. The body weight of broodstocks was between 1.0 and 2.0 kg for each individual.

Figure 1: Spawning compartments inside the reproduction pond.
The spawning (Day 0) occurred the day before the egg collection day (Day 1). It took place naturally and always happened between 5 pm and the sunset at around 6 pm. Fertilized eggs were collected the next morning (Day 1). To collect the eggs, the whole nest constructed from the fibres of sugar palm was taken out of the water from the spawning compartment and put straightaway in a bucket filled with water. The bucket containing the nest and eggs was then moved into the hatchery. Further, the eggs sticking to the nest substrates were released from the nest by gently shaking the nest. The released eggs floating on the water surface were then transferred into round plastic basins with a diameter of 60 cm, filled with water at a depth of 25–30 cm (Figures 2 and 3).

Figure 2: Eggs are transferred from the bucket into the basin.

Figure 3: Basins used for rearing the eggs and larvae in the hatchery.
The eggs were obtained from six pairs of broodstocks that consisted of three categories: the grey broodstocks that spawned the previous month (Grey I.1 and Grey I.2), the grey broodstocks that did not spawn the previous month (Grey II.1 and Grey II.2), and the pink broodstocks that did not spawn the previous month (Pink 1 and Pink 2). Hence, the study was performed in duplicate.
Rearing of eggs and larvae
The eggs originated from the same nest were kept in the same basin. The eggs and larvae were named after the body colour of their respective parents, so the grey larvae were the offspring of grey broodstocks, with Grey I were the larvae whose broodstocks spawned the previous month and Grey II were the larvae whose broodstocks did not spawn the previous month, while the pink larvae were the offspring of pink broodstocks.
Traditionally, the larvae are maintained in the basins for 8 to 9 days during which they are not given any exogenous feed due to the yolk sac that is still present; and at 8 or 9 day old they are transferred into the bigger tanks. For this study, the larvae were reared in the basins for a period until the water environment can no longer support their livelihood. If mass death occurs then the surviving larvae are immediately transferred into the bigger tank, which is the tank of 2 x 1 x 0.6 m3 utilized for the pre-juvenile fish.
The eggs and larvae were reared under ambient water temperature, without aeration, and natural light condition which was about 12 h light and 12 h dark (Figure 3). The sunlight was the only source of light during the day. After sunset, the hatchery was left dark until sunrise. During the study, the larvae were not fed at all, and their nutrition depended only on the egg yolk. The layer of an oily film formed on the water surface was removed to keep the water oxygenated by the atmospheric air. On Day 10, the observation was ended and surviving larvae were counted and removed into the big tanks (pre-juvenile tanks).
Observations and measurements
Samples of eggs and larvae were taken out of the basin and put in a dish for daily photography. Rotten eggs and dead larvae were counted every day and discarded. Water quality parameters measured were pH and water temperature using portable TDS-EC water quality meter, with the measurement taken in the morning and afternoon every other day. There was no water exchange or aeration was performed during the study.
The number of eggs produced by each broodstock was calculated from the sum of the surviving larvae on Day 10 and the total number of deaths. The survival rates of the larvae on Day 9 and Day 10 were calculated according to the formula:
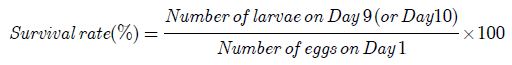
Analysis of variance (ANOVA) single factor using Microsoft Excel 2010 was utilized to determine the significance in the decline of survival rates in the Grey II.1 and Grey II.2 basins and in the difference of the number of deaths in the Grey I.2 basin.
Terminology for gourami development
The terms utilized to describe embryonic, larval, and juvenile stages vary according to different authors, leading to the confusion in defining those stages. In this paper, the terms are defined based on Fujimura and Okada [9] as follow: egg or embryonic stage is the period from fertilization until hatching. Larva is the stage that includes the time right after hatching until the complete absorption of the yolk sac. During this stage, the larvae live without additional exogenous food. The late larval period lasts until the initiation of exogenous feeding. Once the fish starts eating exogenous food, it can no longer be called a larva. The next stage for gourami is fry or pre-juvenile stage, where baby fish starts feeding on exogenous food until it reaches a length of about 5 cm. While the pre-juvenile stage lasts for two to three months depending on the strain and nutrition, the juvenile stage lasts until the fish reaches the first maturation of gametes.
Results
The observation of the early development of both types of gourami Padang strain at ambient water temperature of 25–28°C during the first ten days following fertilization is presented in Figure 4 where the main features of developmental stages are discussed. The number of rotten or unfertilized eggs and dead larvae which constitute the total number of deaths are presented in Table 1, while the number of surviving larvae, the total number of eggs produced, and the survival rates of larvae until Day 9 and Day 10 are presented in Table 2.
| Day | Grey I.1 | Grey I.2 | Grey II.1 | Grey II.2 | Pink 1 | Pink 2 |
|---|---|---|---|---|---|---|
| 1 | 0.0 | 0.0 | 0.0 | 0.0 | 9.3 | 6.0 |
| 2 | 0.2 | 2.6 | 1.6 | 1.2 | 0.5 | 47.5 |
| 3 | 0.6 | 10.0 | 3.2 | 0.6 | 0.8 | 0.6 |
| 4 | 1.5 | 2.0 | 1.9 | 0.5 | 0.7 | 1.6 |
| 5 | 0.7 | 6.4 | 5.0 | 1.4 | 2.3 | 0.4 |
| 6 | 2.3 | 9.4 | 5.6 | 0.6 | 0.3 | 0.6 |
| 7 | 0.5 | 1.7 | 6.8 | 0.6 | 0.4 | 0.0 |
| 8 | 0.5 | 1.7 | 1.6 | 0.8 | 0.7 | 0.1 |
| 9 | 0.6 | 1.2 | 1.2 | 0.2 | 0.0 | 0.0 |
| 10 | 0.2 | 7.9 | 42.8 | 59.9 | 0.1 | 0.4 |
| Total death (%) | 7.1 | 43.2 | 69.8 | 65.9 | 15.2 | 57.2 |
Table 1: Number of rotten eggs and dead larvae (total death) in percentage during the study.
| Grey I.1 | Grey I.2 | Grey II.1 | Grey II.2 | Pink 1 | Pink 2 | |
|---|---|---|---|---|---|---|
| Surviving larvae | 1,518 | 945 | 1,081 | 1,318 | 1,616 | 816 |
| Total eggs | 1,634 | 1,663 | 3,584 | 3,865 | 1,906 | 1,906 |
| Mean of total eggs | 1,649 ± 15 | 3,725 ± 141 | 1,906 ± 0 | |||
| SR D9 (%) | 93.1 | 64.8 | 72.9 | 94.0 | 84.9 | 43.2 |
| SR D10 (%) | 92.9 | 56.8 | 30.2 | 34.1 | 84.8 | 42.8 |
Table 2: Number of surviving larvae, total eggs, and survival rates (SR) on Day 9 and Day 10.
Explanations of the embryos and larvae development presented in Figure 4 are as follow: Day 1 was the first day when the eggs were placed in the basins. The eggs of gourami were spherical and yellowish-orange in colour. Some newly fertilized eggs began to take an ovoid shape, while the others were still perfectly round. On Day 1, the eggs (or embryos) moved around with twitching-like movements. Using naked eyes we could not differentiate the grey eggs from the pink ones. These eggs with a diameter of around 2.5 mm were considered as large eggs. Giant gourami parents and other Osphronemus spp. are categorized as guarders. They are socalled because the eggs and young are guarded naturally by one or both of the parents. They produce relatively few eggs which are larger than those of non-guarders [10].
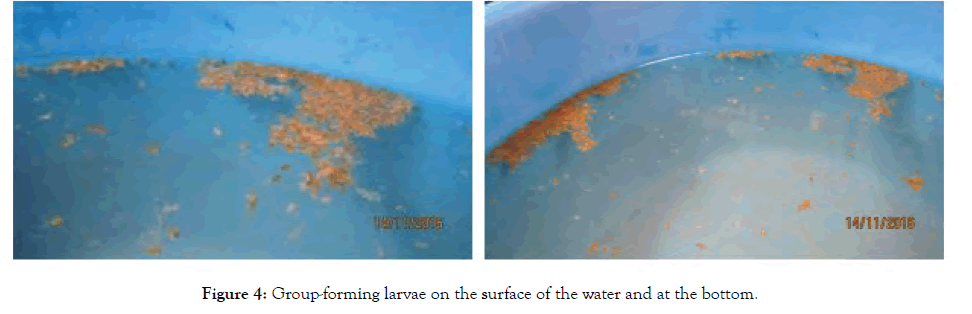
Figure 4: Group-forming larvae on the surface of the water and at the bottom.
On Day 2 (36–48 h after fertilization, AF), the majority of the grey eggs hatched. This can be seen from the tail (notochord) that was formed at the end of the ovoid embryo, while for the pink eggs the hatching did not occur yet since the tail could not be seen at all eggs. Termvidchakorn & Hortle [10] also found that the floating eggs of gourami hatched in 36 hours at 25–30°C. The embryos at hatching were completely transparent with no visible pigmentation. The hatched gourami embryo was then called larva with visible tail projected from one end of the ovoid yolk sac.
On Day 3, both grey and pink larvae were swimming actively and the eye formation started for some grey larvae, while the others were still in the stage of eyeless tailed larvae. On the other hand, all pink larvae hatched that can be seen from the presence of the tail and some had eyes. The pigmentation of the eyes in the grey larvae was very distinctive, while in the pink larvae the eye colour was less prominent. The retina in the grey larvae contained pigment earlier than in the pink larvae. The pigmentation of retina apparently completed within 24 hours since from Day 4 both grey and pink larvae had dark eye colour.
On Day 4, it can be seen that the larvae are swimming upside down or sideways and the black eye colour is very clear for both strains. On Day 5 the size of embryo increases while the size of yolk sac reduced. On Day 6 in the grey larvae, melanophores become visible which is not existent in the pink larvae. All larvae are swimming normally (upside up). On Day 7, the difference in pigmentation between grey and pink larvae starts to become visible. On Day 8, this difference is very clear that the grey larvae show a distinctive dark colour, while the pink larvae stay orange colour. On Day 9 and Day 10, the difference in colour between the two types is even more visible. In addition, on Day 10, the remnants of the yolk sac are still visible in some larvae.
From the pictures in Figure 4, it can also be seen that the Grey II.1 larvae are all grey in colour, while the Grey II.2 larvae have a mixture of grey and pink-orange colour. However, we did not take any pictures of the Grey I.2 and Grey II.1 larvae past Day 7. Therefore, we cannot tell their respective colour. Similar with the grey larvae, the Pink 1 larvae are composed of a mixture of pink and grey larvae, while the Pink 2 larvae are all pink-orange in colour.
Embryos showed twitching movements from Day 1 and they remained in groups on the surface of the water. On the other hand, the larvae began to swim actively since newly hatched when the tail appeared. They stayed in groups, mostly on the surface of the water, but some lay on the bottom of the basin. No schooling was noted but they appeared to form groups of 30–250 individuals (Figure 5). In addition, no cannibalism was observed among these larvae.
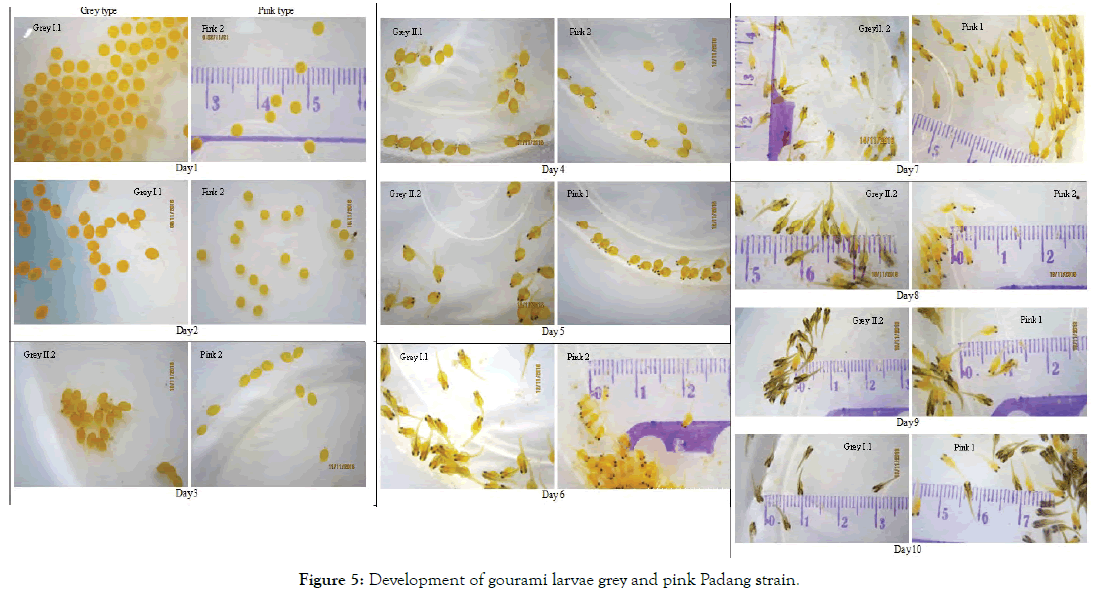
Figure 5: Development of gourami larvae grey and pink Padang strain.
On Day 10, the number of dead larvae was significantly high in the Grey II.1 and Grey II.2 basins that reached respectively 58.6% and 63.7% of the remaining population. Therefore, the experiment was stopped immediately and the surviving larvae were transferred into the bigger tanks.
Discussion
The conditions of hatching and growth, as well as survival rate, are the principal parameters for the determination of successful larval rearing. The environment and water quality may be different for gourami larvae in aquaculture compared to gourami larvae in natural habitats such as river and lake. Rearing environment and water quality among different hatcheries may also vary. One of the key regulators of metabolism and growth is temperature because it plays an important role in larval physiology and biochemistry through ontogenesis [7]. In this study, the ambient water temperature ranged from 25°C in the morning to reach a maximum of 28°C in the afternoon. We observed that the gourami growers in Central Java utilized an enclosed pond as hatchery similar to a greenhouse to keep the temperature high (>30°C) in order to accelerate larval development. However, this system has a disadvantage that there is no means to control the water temperature once it gets too high during the sunny day due to lack of ventilation. The range of temperature used in this study seems to support larval development well enough which can be seen from the normal development as stated by Kimmel et al., Sanches et al., Honji et al., and Kratochwil et al. [11-13].
The hatching period of gourami in this study at ambient water temperature was first observed on Day 2 (36–48 h AF) and was completed on Day 3 (60–72 h AF). This is similar to non-migratory fish species such as Hoplias malabaricus, which hatched 44 h AF at 24–26.5°C [12] and zebra fish Danio rerio that hatched 48–72 h AF [11]. Although the hatching was later than the grey larvae, individuals of pink larvae hatched sporadically throughout the third day of development. After hatching, the larvae fed on the egg yolk for 5–7 days, as also reported by Termvidchakorn and Hortle [10].
Temperature has a positive influence on developmental rate of larvae as shown in Midas cichlid, Amphilophus xiloaensis and in zebra fish Danio rerio, where there was an increase of embryonic development rate of 1.76-fold and 1.42-fold respectively when temperature was raised from 25 to 31°C [11,13]. The positive influence of temperature is not only seen in tropical fish, but also in high longitude fish such as burbot Lota lota, where the embryogenesis and hatching period significantly decreased as temperature increased, from 53 and 19 days at 2°C to 25 and 7 days at 6°C respectively [14].
This study showed a mortality rate of 9.3% and 6.0% on Day 1 for Pink 1 and Pink 2 eggs (Table 1), which is thought to be related to the unfertilized eggs. Furthermore, on Day 2 the Pink 2 eggs experienced significantly high mortality (47.5%). This is thought to be caused by the poor quality of eggs related to the broodstock quality since it was not assessed individually before the spawning. From fertilization to the onset of feeding on the planktons, embryos and larvae are dependent on the nutrition reserves from the egg yolk. The quality of these reserves depends on the quality of food ingested by the female during oogenesis and maturation. It has been shown that egg quality is influenced by the age, energy reserves, and environmental condition of females during the pre-spawning period [15-17]. Also, the egg size is correlated with larval size [18]. Despite their smaller size, eggs from low-fecundity young female have a better quality than eggs from older female [19]. Furthermore, food availability and intensity of social interactions experienced by females prior to and during spawning will significantly determine the volume of yolk reserves and somatic development of larvae at hatching [20]. Therefore, to obtain consistently eggs and larvae of high quality, it is important to give more attention to possible parental factors that can affect larval and juvenile quality. Broodstock welfare and nutrition are regarded as key factors. However, the role of these on subsequent larval quality still needs to be addressed [7].
Although carried out in duplicate, the study showed a great difference between the two replications, as seen in the number of total death between the Grey I.1 and Grey I.2 larvae, and between the Pink 1 and Pink 2 larvae. Therefore, statistical analysis is carried out individually for each category of larvae to avoid an average value that does not reflect the actual condition (Tables 1 and 2).
In the traditional gourami culture, the larvae only feed on the egg yolk for the first eight days. They start eating exogenous food such as water fleas after being transferred into the bigger tanks. Therefore, there was a period of combination between endogenous and exogenous food supply for two to three days following the transfer since the remnants of the yolk sac are still visible in some larvae on Day 10 (Figure 4). This is in accordance with Termvidchakorn & Hortle [10] who stated that after hatching, the larvae feed on the egg yolk for 5–7 days, and after the yolk sac has been absorbed, they feed on zooplankton. Irrespectively to their size, in general, fish larvae including gourami, completely absorb the yolk sac after ten days following the fertilization [9,10,14,21,22].
Contrarily to the terminology that utilizes larvae nomenclature for the fries feeding on exogenous feed [8,12], the terminology utilized in this study defined that once the fish starts eating exogenous food, it can no longer be called a larva. This is similar to the staging applied for Nile tilapia Oreochromis niloticus by Fujimura and Okada [9] where the late larval period lasts until the initiation of exogenous feeding. In gourami, the larval period is followed by the pre-juvenile stage or fry that lasts for two to three month. Then, the juvenile stage lasts until the fish reaches maturity.
Groups of larvae consisting of 30–250 individuals were observed at the surface of the water and at the bottom of the basins (Figure 5). This group formation is thought to correlate with the development of group cohesion or aggregative behaviour and group communication, as no study has yet been undertaken on these behaviours in gourami.
For the Grey II.1 and Grey II.2 basins that experienced the mass death on Day 10, the second highest number of deaths occurred in the Grey II.1 basin on Day 7 with 244 deaths or 8.2% of the remaining population in the basin. The daily death of less than 10% is acceptable and considered normal. On the contrary, on Day 10 the numbers of deaths in the Grey II.1 and Grey II.2 basins were 1,533 individuals or 58.6% and 2,315 individuals or 63.7% of the remaining population. The number of deaths greater than 50% of the population indicates that at least one parameter in the system failed to work as it should. In this case, the toxic substances accumulated in the basin and originated only from the larval excretion were suspected to be the cause.
In this study, metabolites consisting of compounds such as ammonia (NH3), ammonium (NH4 +), nitrite (NO2 -), and CO2 came solely from the reared larvae. Moreover, the basins used for larval rearing have been washed before use, so the aerobic nitrification bacteria were not well established yet. Due to the non-existing water flow system and non-established aerobic nitrifying bacteria in the basins, the total ammonia from larval excretion would have accumulated in the water during the first seven days of rearing to reach its pic concentration on Day 7 [23]. From Day 7 to Day 10, ammonia-oxidizing bacteria which were now established started producing nitrite, while the concentration of total ammonia was decreasing from its maximum value. In this study, the fish death occurred between dusk on Day 9 and early morning on Day 10, in which the concentration of total ammonia was still high and the concentration of nitrite has started to rise. This combination was more likely to cause mass death in the Grey II.1 and Grey II.2 basins. The death of the fishes occurred at the ammonia concentration of 0.5 mg/L [24], or at the combination of total ammonia concentration of 4.43 mg/L and nitrite concentration of 1.95 mg/L [25].
On Day 9, the Grey II.1 and Grey II.2 basins had a high biomass density of 31 and 43 larvae/L, while in the Grey I.1, Grey I.2, Pink 1, and Pink 2 basins the biomass density was much lower (18, 13, 19, 10 larvae/L respectively). For the basins with low biomass density (<20 larvae/L), these metabolites were not considered as a threat, shown by the low number of deaths (<0.5%) in these basins on Day 10, except for the Grey I.2 basin in which the number of deaths reached 7.9% that day (Table 1). Furthermore, the deaths occurring in the Grey I.2 basin on Days 3, 5, 6, and 10 (Table 1) were significantly higher than the other days (p<0.05). On the other hand, the number of deaths in the Grey I.1 basin which was the duplicate of the Grey I.2 basin remained low on these Days 3, 5, 6, and 10 (Table 1). Therefore, it is more likely that the high number of deaths is not only caused by the high biomass density that leads to the high concentration of toxic substances, but also by the condition and quality of larvae. The larvae produced by the Grey I.2 broodstock are significantly in much lower quality and survival rate (p<0.05) than the larvae produced by the Grey I.1 broodstock (Table 2). Similar reasoning applies for the Pink 1 larvae compared to the Pink 2 larvae.
The toxic substances originating from the reared larvae were the only culprits to cause mass mortality in the Grey II.1 and Grey II.2 basins on Day 10. Despite no measurement of the concentrations of ammonia and nitrite was taken, it is highly possible that the high density of larvae led to higher concentrations of ammonia and nitrite in the Grey II.1 and Grey II.2 basins. On Day 10 they reached the toxic levels and caused mass mortality in both basins. This shows the justification of the practices of transferring the larvae on Day 8 or Day 9 from the basins that have been applied by the gourami breeders. However, for a density of fewer than 20 larvae/L, it seems that the larval rearing until Day 10 in such a basin will cause no problem (Table 1).
The Grey I.1 and Grey I.2 females have spawned the previous month while the Grey II.1 and Grey II.2 females did not spawn the previous month but they spawned two months earlier. It is a common knowledge among the gourami growers that the fecundity of female gourami will decrease in the second month if they spawn two consecutive months. This study showed that the fecundity of Grey I females was only 44.3% of Grey II females. Thus, consecutive spawning will reduce fecundity in gourami. However, further studies need to be done to confirm this finding.
The smaller quantity of eggs released by the pink female gourami may reflect the gonado-somatic index values, which increase during the final gonadal maturation. Thus, macroscopic and microscopic analysis of the gonads in the future may help explain the small quantity of the released eggs.
Figure 4 shows that on Day 7 the body colours of Grey 2 and Pink 1 larvae began to differentiate into two colours: bright orange and darker orange. This colour difference is very clear starting from Day 9. This difference in colours is thought to be related to the broodstocks that were produced as a result of crossbreeding between the two colours, as it is a common practice carried out by the fish growers. Therefore, the broodstocks that phenotypically appear as pink could have a grey genotype or vice versa.
Conclusion
The conditions of hatching and growth, as well as survival rate, are the principal parameters for the determination of successful larval rearing. The environment and water quality may be different for gourami larvae in aquaculture compared to gourami larvae in natural habitats such as river and lake. Rearing environment and water quality among different hatcheries may also vary. One of the key regulators of metabolism and growth is temperature because it plays an important role in larval physiology and biochemistry through ontogenesis [7]. In this study, the ambient water temperature ranged from 25°C in the morning to reach a maximum of 28°C in the afternoon. We observed that the gourami growers in Central Java utilized an enclosed pond as hatchery similar to a greenhouse to keep the temperature high (>30°C) in order to accelerate larval development. However, this system has a disadvantage that there is no means to control the water temperature once it gets too high during the sunny day due to lack of ventilation. The range of temperature used in this study seems to support larval development well enough which can be seen from the normal development as stated by Kimmel et al., Sanches et al., Honji et al., and Kratochwil et al. [11-13].
The hatching period of gourami in this study at ambient water temperature was first observed on Day 2 (36–48 h AF) and was completed on Day 3 (60–72 h AF). This is similar to non-migratory fish species such as Hoplias malabaricus, which hatched 44 h AF at 24–26.5°C [12] and zebra fish Danio rerio that hatched 48–72 h AF [11]. Although the hatching was later than the grey larvae, individuals of pink larvae hatched sporadically throughout the third day of development. After hatching, the larvae fed on the egg yolk for 5–7 days, as also reported by Termvidchakorn and Hortle [10].
Temperature has a positive influence on developmental rate of larvae as shown in Midas cichlid, Amphilophus xiloaensis and in zebra fish Danio rerio, where there was an increase of embryonic development rate of 1.76-fold and 1.42-fold respectively when temperature was raised from 25 to 31°C [11,13]. The positive influence of temperature is not only seen in tropical fish, but also in high longitude fish such as burbot Lota lota, where the embryogenesis and hatching period significantly decreased as temperature increased, from 53 and 19 days at 2°C to 25 and 7 days at 6°C respectively [14].
This study showed a mortality rate of 9.3% and 6.0% on Day 1 for Pink 1 and Pink 2 eggs (Table 1), which is thought to be related to the unfertilized eggs. Furthermore, on Day 2 the Pink 2 eggs experienced significantly high mortality (47.5%). This is thought to be caused by the poor quality of eggs related to the broodstock quality since it was not assessed individually before the spawning. From fertilization to the onset of feeding on the planktons, embryos and larvae are dependent on the nutrition reserves from the egg yolk. The quality of these reserves depends on the quality of food ingested by the female during oogenesis and maturation. It has been shown that egg quality is influenced by the age, energy reserves, and environmental condition of females during the pre-spawning period [15-17]. Also, the egg size is correlated with larval size [18]. Despite their smaller size, eggs from low-fecundity young female have a better quality than eggs from older female [19]. Furthermore, food availability and intensity of social interactions experienced by females prior to and during spawning will significantly determine the volume of yolk reserves and somatic development of larvae at hatching [20]. Therefore, to obtain consistently eggs and larvae of high quality, it is important to give more attention to possible parental factors that can affect larval and juvenile quality. Broodstock welfare and nutrition are regarded as key factors. However, the role of these on subsequent larval quality still needs to be addressed [7].
Although carried out in duplicate, the study showed a great difference between the two replications, as seen in the number of total death between the Grey I.1 and Grey I.2 larvae, and between the Pink 1 and Pink 2 larvae. Therefore, statistical analysis is carried out individually for each category of larvae to avoid an average value that does not reflect the actual condition (Tables 1 and 2).
In the traditional gourami culture, the larvae only feed on the egg yolk for the first eight days. They start eating exogenous food such as water fleas after being transferred into the bigger tanks. Therefore, there was a period of combination between endogenous and exogenous food supply for two to three days following the transfer since the remnants of the yolk sac are still visible in some larvae on Day 10 (Figure 4). This is in accordance with Termvidchakorn & Hortle [10] who stated that after hatching, the larvae feed on the egg yolk for 5–7 days, and after the yolk sac has been absorbed, they feed on zooplankton. Irrespectively to their size, in general, fish larvae including gourami, completely absorb the yolk sac after ten days following the fertilization [9,10,14,21,22].
Contrarily to the terminology that utilizes larvae nomenclature for the fries feeding on exogenous feed [8,12], the terminology utilized in this study defined that once the fish starts eating exogenous food, it can no longer be called a larva. This is similar to the staging applied for Nile tilapia Oreochromis niloticus by Fujimura and Okada [9] where the late larval period lasts until the initiation of exogenous feeding. In gourami, the larval period is followed by the pre-juvenile stage or fry that lasts for two to three month. Then, the juvenile stage lasts until the fish reaches maturity.
Groups of larvae consisting of 30–250 individuals were observed at the surface of the water and at the bottom of the basins (Figure 5). This group formation is thought to correlate with the development of group cohesion or aggregative behaviour and group communication, as no study has yet been undertaken on these behaviours in gourami.
For the Grey II.1 and Grey II.2 basins that experienced the mass death on Day 10, the second highest number of deaths occurred in the Grey II.1 basin on Day 7 with 244 deaths or 8.2% of the remaining population in the basin. The daily death of less than 10% is acceptable and considered normal. On the contrary, on Day 10 the numbers of deaths in the Grey II.1 and Grey II.2 basins were 1,533 individuals or 58.6% and 2,315 individuals or 63.7% of the remaining population. The number of deaths greater than 50% of the population indicates that at least one parameter in the system failed to work as it should. In this case, the toxic substances accumulated in the basin and originated only from the larval excretion were suspected to be the cause.
In this study, metabolites consisting of compounds such as ammonia (NH3), ammonium (NH4 +), nitrite (NO2 -), and CO2 came solely from the reared larvae. Moreover, the basins used for larval rearing have been washed before use, so the aerobic nitrification bacteria were not well established yet. Due to the non-existing water flow system and non-established aerobic nitrifying bacteria in the basins, the total ammonia from larval excretion would have accumulated in the water during the first seven days of rearing to reach its pic concentration on Day 7 [23]. From Day 7 to Day 10, ammonia-oxidizing bacteria which were now established started producing nitrite, while the concentration of total ammonia was decreasing from its maximum value. In this study, the fish death occurred between dusk on Day 9 and early morning on Day 10, in which the concentration of total ammonia was still high and the concentration of nitrite has started to rise. This combination was more likely to cause mass death in the Grey II.1 and Grey II.2 basins. The death of the fishes occurred at the ammonia concentration of 0.5 mg/L [24], or at the combination of total ammonia concentration of 4.43 mg/L and nitrite concentration of 1.95 mg/L [25].
On Day 9, the Grey II.1 and Grey II.2 basins had a high biomass density of 31 and 43 larvae/L, while in the Grey I.1, Grey I.2, Pink 1, and Pink 2 basins the biomass density was much lower (18, 13, 19, 10 larvae/L respectively). For the basins with low biomass density (<20 larvae/L), these metabolites were not considered as a threat, shown by the low number of deaths (<0.5%) in these basins on Day 10, except for the Grey I.2 basin in which the number of deaths reached 7.9% that day (Table 1). Furthermore, the deaths occurring in the Grey I.2 basin on Days 3, 5, 6, and 10 (Table 1) were significantly higher than the other days (p<0.05). On the other hand, the number of deaths in the Grey I.1 basin which was the duplicate of the Grey I.2 basin remained low on these Days 3, 5, 6, and 10 (Table 1). Therefore, it is more likely that the high number of deaths is not only caused by the high biomass density that leads to the high concentration of toxic substances, but also by the condition and quality of larvae. The larvae produced by the Grey I.2 broodstock are significantly in much lower quality and survival rate (p<0.05) than the larvae produced by the Grey I.1 broodstock (Table 2). Similar reasoning applies for the Pink 1 larvae compared to the Pink 2 larvae.
The toxic substances originating from the reared larvae were the only culprits to cause mass mortality in the Grey II.1 and Grey II.2 basins on Day 10. Despite no measurement of the concentrations of ammonia and nitrite was taken, it is highly possible that the high density of larvae led to higher concentrations of ammonia and nitrite in the Grey II.1 and Grey II.2 basins. On Day 10 they reached the toxic levels and caused mass mortality in both basins. This shows the justification of the practices of transferring the larvae on Day 8 or Day 9 from the basins that have been applied by the gourami breeders. However, for a density of fewer than 20 larvae/L, it seems that the larval rearing until Day 10 in such a basin will cause no problem (Table 1).
The Grey I.1 and Grey I.2 females have spawned the previous month while the Grey II.1 and Grey II.2 females did not spawn the previous month but they spawned two months earlier. It is a common knowledge among the gourami growers that the fecundity of female gourami will decrease in the second month if they spawn two consecutive months. This study showed that the fecundity of Grey I females was only 44.3% of Grey II females. Thus, consecutive spawning will reduce fecundity in gourami. However, further studies need to be done to confirm this finding.
The smaller quantity of eggs released by the pink female gourami may reflect the gonado-somatic index values, which increase during the final gonadal maturation. Thus, macroscopic and microscopic analysis of the gonads in the future may help explain the small quantity of the released eggs.
Figure 4 shows that on Day 7 the body colours of Grey 2 and Pink 1 larvae began to differentiate into two colours: bright orange and darker orange. This colour difference is very clear starting from Day 9. This difference in colours is thought to be related to the broodstocks that were produced as a result of crossbreeding between the two colours, as it is a common practice carried out by the fish growers. Therefore, the broodstocks that phenotypically appear as pink could have a grey genotype or vice versa.
Acknowledgement
This study was supported by the Indonesian Ministry of Research, Technology, and Higher Education under the Program Insentif Riset Sinas (Grant number: 318/SP2H/LT/DRPM/VI/2016). I sincerely thank Rono Rahmat (Baron) for the excellent assistance during the harvests of the eggs and Pak Jhonly Pilo for allowing me experimenting on the eggs and larvae and working on his farm for this study.
REFERENCES
- Rainboth WJ. Fishes of the Cambodian Mekong. (V. H. Niem, K. E. Carpenter, & N. De Angelis, Eds.) (FAO species). Rome. 1996.
- Weber M, de Beaufort L. The Fishes of the Indo-Australian Archipelago IV. Leiden: E. J. Brill Ltd. 1922.
- Murray AM, Zaim Y, Rizal Y, Aswan Y, Gunnell GF, Ciochon RL. A fossil gourami (Teleostei, Anabantoidei) from probable Eocene deposits of the Ombilin Basin, Sumatra, Indonesia. J Vertebr Paleontol. 2015; 35.
- Surya Mina Farm. Ikan Gurame Adalah Ikan Yang Mahal, Mengapa? 2015.
- Tanjung LR, Said DS, Maghfiroh M. Ikan Gurami (Osphronemus gouramy) Strain Padang Terbukti Memiliki Ketahanan Alami terhadap Infeksi Aeromonas. Proceeding of Konferensi Akuakultur Indonesia. 2013; 96–107.
- Cahu C, Zambonino-Infante JL. Formulated Diets Replacement for Live Food in Marine Fish Larvae. Aquaculture. 2001; 200: 161–180.
- Helvick J, Hamre K, Hordvik I, Meeran VT, Ressem H, Schartl M. The fish larva : a transitional life form, the foundation for aquaculture and fisheries. The Research Council of Norway. 2009.
- Çelik İ, Çelik P, Gürkan M, Şahin T. Larval Development of The Freshwater Angelfish Pterophyllum scalare (Teleostei: Cichlidae). Turk J Fish Aquat Sci. 2014; 14: 863–874.
- Fujimura K, Okada N. Development of the embryo, larva and early juvenile of Nile tilapia Oreochromis niloticus ( Pisces : Cichlidae ). Develop Growth Differ. 2007; 49: 301–324.
- Termvidchakorn A, Hortle KG. A guide to larvae and juveniles of some common fish species from the Mekong River Basin. MRC Technical Paper. 2013.
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of Embryonic Development of the Zebrafish. Develop Dyn. 1995; 203: 253–310.
- Honji RM, Tolussi CE, Mello PH, Caneppele D, Moreira RG. Embryonic development and larval stages of Steindachneridion parahybae (Siluriformes: Pimelodidae) - implications for the conservation and rearing of this endangered Neotropical species. Neo Ichthyology. 2012; 10: 313–327.
- Kratochwil CF, Sefton MM, Meyer A. Embryonic and larval development in the Midas cichlid fish species flock (Amphilophus spp.): A new evo-devo model for the investigation of adaptive novelties and species differences. BMC Develop Bio. 2015; 15: 1–15.
- Lahnsteiner F, Kletzl M, Weismann T. The effect of temperature on embryonic and yolk-sac larval development in the burbot Lota lota. J Fis Bio. 2012; 81: 977–986.
- Chambers RC, Leggett WC. Maternal Influences on Variation in Egg Sizes in Temperate Marine Fishes. Amer Zool. 1996; 36: 180–196.
- Ouellet P, Lambert Y, Be I. Cod egg characteristics and viability in relation to low temperature and maternal nutritional condition. ICES J Mar Sci. 2001; 58: 672–686.
- Bobe J. Egg quality in fish : Present and future challenges. Ani Frons. 2015; 5: 66–72.
- Hinckley S. Variation of Egg Size of Walleye Pollock Theragra chalcogramma with a Preliminary Examination of the Effect of Egg Size on Larval Size. Fis Bull. 1990; 88: 471–483.
- Kjorsvik E, Mangor-Jensen A, Holmefjord I. Egg Quality in Fishes. Adv Mar Bio. 1990; 26: 71–113.
- Kerrigan B. Variability in larval development of the tropical reef fish Pomacentrus ambionensis (Pomacentridae): the parental legacy. Mar Bio. 1997; 127: 395–402.
- Groth WO. Embryology of the siamese fighting fish Betta splendens. 1970.
- Lee SH, Kim CC, Koh SJ, Shin LS, Cho JK, Han KH. Egg Development and Morphology of Larva and Juvenile of the Oryzias latipes. Dev & Rep. 2014; 18: 173–178.
- Aquaportail. Définition de cyclage aquarium. https://www.aquaportail.com/definition-13717-cyclage-aquarium.html. Dernière mise à jour le: 2016.
- Ogbonna JF, Chinomso AA. Determination of the Concentration of Ammonia that Could Have Lethal Effect on Fish Pond. ARPN J Eng App Sci. 2010; 5: 1–5.
- Svobodová Z, Máchová J, Poleszczuk G, Huda J, Hamácková J, Kroupová H. Nitrite poisoning of fish in aquaculture facilities with water- recirculating systems: Three case studies. Acta Vet Brno. 2015; 74: 129–137.
Citation: Tanjung LR (2019) Survival Rate and Early Development of Giant Gourami Padang Strain Osphronemus gouramy (Perciformes: Osphronemidae). 10:576. doi: 10.35248/2155-9546.19.10.576
Copyright: © 2019 Tanjung LR. This is an open access article distributed under the term of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Sources of funding : This study was supported by the Indonesian Ministry of Research, Technology, and Higher Education under the Program Insentif Riset Sinas (Grant number: 318/SP2H/LT/DRPM/VI/2016). I sincerely thank Rono Rahmat (Baron) for the excellent assistance during the harvests of the eggs and Pak Jhonly Pilo for allowing me experimenting on the eggs and larvae and working on his farm for this study.