PMC/PubMed Indexed Articles
Indexed In
- Open J Gate
- Academic Keys
- JournalTOCs
- ResearchBible
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 8, Issue 1
Subjective Cognitive Decline: Mental Health, Loneliness, Pain and Quality Of Life: Poblational Study
Pedro Montejo Carrasco1*, David Prada Crespo1,2, Eduardo Pedrero-P�?©rez3 and Mercedes Montenegro-Peña42Department of Experimental Psychology, Faculty of Psychology, Complutense University, Madrid, Spain
3Department of Assessment and Quality Formation and Research Unit, Madrid Salud, Madrid City Council, Complutense University, Madrid, Spain
4Dirección General de Mayores. General Directorate for the Elderly, Families, Equality and Social Welfare Governing Area, Madrid City Council, Spain
Received: 16-Dec-2019 Published: 13-Jan-2020, DOI: 10.35248/2329-8847.20.8.456
Abstract
Background: Subjective cognitive decline is considered to be a risk for Alzheimer’s disease. However, it can also be associated with non-cognitive variables.
Objectives: This study analyzes the association between subjective cognitive decline (SCD) and variables related to memory, mental health, morbidity, pain, quality of life, loneliness, lifestyle, and social aspects; analyzes predictors of SCD.
Methods: Cross-sectional epidemiological study of a sample of individuals randomly selected from a city census. Telephone interviews were conducted with 1775 individuals aged over 55 years. We administered a 7-item questionnaire on SCD and asked about health, lifestyle, and social variables; we also administered a measure of general mental health, the Goldberg Health Questionnaire, and the health-related quality of life scale COOP/ WONCA.
Results: SCD showed statistically significant associations with orientation in time (r=0.16), mental health variables (r=0.41), quality of life (r=0.36), loneliness (Eta2=0.04), disability (R2=0.05), pain (R2=0.12), hearing difficulties (R2=0.03), vision problems (R2=0.05), and chronic disease (R2=0.04). The variables orientation in time, mental health, depression, sleep quality, multimorbidity, and hearing difficulties were identified as predictors of SCD (p<0.001; R2=0.30).
Conclusion: The heterogeneity of the variables associated with SCD should be taken into account to differentiate individuals at increased risk of developing Alzheimer’s disease from those in whom the condition may be explained by other factors.
Keywords
Subjective cognitive decline; Memory complaints; Loneliness; Pain; Mental health; Quality of life
Introduction
The Subjective Cognitive Decline (SCD) refers to a subjective experience of decline of certain cognitive functions, regardless of actual performance. Objective performance may be normal or show subtle decline that may not be detected by common tests. While SCD may have numerous causes, current interest in the concept centers on its role as a potential predictor of Alzheimer’s disease (AD). The Subjective Cognitive Decline Initiative (SCD-I) Working Group was created in 2012 to develop the concept and all related aspects [1]; The SCD-I established a series of criteria for SCD and for “SCD plus,” which denotes SCD with increased likelihood of preclinical AD: subjective decline, involving memory more than other domains; onset within the last five years; age of onset at 60 years or older; concern about memory failures; feeling of poorer performance than others of the same age; and, where possible, confirmation from an informant and APOE genotype and biomarker evidence. Researchers have studied numerous AD biomarkers associated with SCD, including brain hypometabolism [2] and amyloid accumulation [3]. Biomarkers are studied through imaging, laboratory, and genetic studies, as well as other techniques [4]. Neuropsychological markers constitute another line of research [5].
Given that the established criteria for SCD include “subtle or no cognitive impairment,” it may seem strange that one of the most important questions in this area is to what extent SCD is associated with an objectively measured decrease in memory performance. Fonseca et al. [6] report a lack of correlation between SCD and objective memory performance, arguing that such an association is not needed as normal cognition is maintained due to compensatory mechanisms. However, authors including Koppara et al. [7] have found that baseline performance in individuals with SCD is poorer than that of controls, and that the subsequent decline is more pronounced in the former group. Furthermore, Slavin et al. [8] report greater effect sizes for psychological factors than for cognitive performance in explaining cognitive complaints (multiple regression analyses, R2=0.127 vs. R2=0.040), questioning the usefulness of subjective cognitive complaints for predicting cognitive impairment. More research is needed in this field: while the issue of Subjective Memory Complaints (SMCs) is addressed in numerous studies [9], few authors refer to the new SCD framework [10].
The factor most reliably associated with SCD is mental health, particularly such aspects as depression and anxiety (with depression showing a stronger association). Certain other variables may be associated with SCD/SMCs through their interaction with depression. The temporal and causal direction of this relationship is not well understood, and may vary by individual. This association has been demonstrated both in clinical and in community samples [11].
SCD has also been linked to health-related quality of life (HRQoL). In a review on Subjective Cognitive Impairment (SCI) and HRQoL, Hill et al. [12] report an association between greater SCI frequency and severity and lower quality of life. According to Scholtissen-In de Braek, Hurks, van Boxtel, Dijkstra & Jolles [13], attentional complaints (another important cognitive area involved in the concept of SCD) in the healthy population are associated with various aspects of HRQoL, including emotional problems, vitality, and social function, as well as depression, anxiety, and sleep quality. Multimorbidity and chronic conditions have also been associated with SCD. Caracciolo et al. [14] conducted a large study with a sample of 11379 twins in Sweden and found a dose-dependent relationship between chronic diseases and SCI, particularly in cases of multimorbidity (defined as >2 diseases). Other study group has previously found memory complaints to be associated with multimorbidity and chronic disease [15]. However, other authors report conflicting findings [16].
Reasons for this heterogeneity include differences in study methodology (cross-sectional, longitudinal, clinical, and population studies) and sample selection (age, randomization, consecutive sampling). The instruments used to assess SCD are also an important factor in explaining discrepancies [17]. As is the case for SMCs, diagnosing SCD presents several challenges [18]. Rabin et al. [19] conducted a review and analysis of the instruments and questions used in different studies, evaluating each of the items commonly used in questionnaires. However, most instruments address only SMCs and do not use the SCD framework. The SCD-I Working Group [20] addresses the main issues surrounding inclusion and exclusion criteria, the role of informants, and other topics relevant in operationalizing the concept of SCD in research and facilitating early detection of individuals at risk of dementia. Several questionnaires for assessing SCD have also been published [21-23].
Our work is intended to be a contribution to population-based research on the SCD. The 2017 City of Madrid Health Survey, a population study based on the city’s census, included several questions analyzing the population’s characteristics in relation to SCD. The objectives of our study were as follows:
1) To analyze the association between SCD and the following health and sociodemographic variables: age, sex, level of schooling, work performance, cognitive performance (orientation in time), quality of life, perceived health status, depression, anxiety, number of diseases, pain, and disability.
2) To analyze the association between SCD and the following social and lifestyle variables: perceived and actual isolation, dependence, alcohol and tobacco consumption, and physical activity.
3) To determine the predictors of SCD.
Materials and Methods
We performed a cross-sectional, descriptive, analytical epidemiological study with a sample taken from the 2017 City of Madrid Health Survey. Participants were selected from the city census using stratified random sampling by administrative district, and the household member to be interviewed was subsequently selected through probability sampling taking into account age and sex. The aim of the survey was to gather information on various health-related factors relevant to the city’s population. Data were collected on sociodemographic and economic variables, lifestyle, use of healthcare services, diseases, diet, and medication; and on other factors including perceived environmental noise, discrimination, and pet ownership. The survey was conducted via telephone interviews. The reference population was composed of residents of Madrid living in their own homes, (ie, a noninstitutionalized population). The total sample comprised 8845 individuals aged between 15 and 98 years. The present study includes only individuals aged over 55: a total of 1775 residents of Madrid, aged between 55 and 98, with a mean age of 68 (standard deviation [SD]=9.35); 42% of participants were men. There was no replacement for those individuals who after three attempts could not be contacted or who were not willing to participate. Therefore, this study does not assess prevalence in the general population (see Limitations section) (Figure 1).
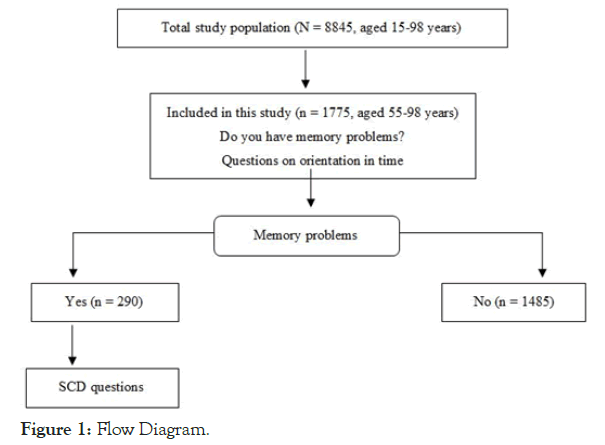
Figure 1. Flow Diagram.
Measurements
Participants were asked several questions about cognitive complaints. The first (“Do you have memory problems?”), with two possible answers (yes/no), was put to all participants aged over 55. Those answering “yes” were asked a further six questions based on the SCD and SCD plus criteria; all were yes/ no questions, with the exception of question 7, with three options (<2 years, 2-5 years, and >5 years); for the purposes of our study, responses to this question were categorized as up to five years (1 point) and more than five years (0 points). These seven questions were synthesized into a scalar variable (the “SCD-7 index”) that draws on fundamental aspects of SCD as described by Jessen et al. [1] and the SCD-I Working Group [20].
Cognitive performance was objectively assessed with five questions on orientation in time: day, date, month, season, and year. These questions are taken from the Mini–Mental State Examination (MMSE) [24] and measure several cognitive areas, particularly memory. Orientation in time is associated with episodic and working memory [25], and shows a positive correlation with total MMSE score [26]. The global score for the variable was the sum of the errors made by each respondent.
General mental health was measured using the Goldberg General Health Questionnaire [27], which is available in 60-, 30-, 28-, and 12-item versions; we used the 12-item questionnaire (GHQ-12) [28]. HRQoL was assessed with the COOP/WONCA questionnaire [29], with nine items from the full version used as verbal stimuli. The validity and reliability of this format have been demonstrated in the Spanish population [30]. Depression, anxiety, and other diseases were assessed with questions phrased “has your doctor told you that you have […]?”; this form of survey is common in population studies [31]. Other health and social variables were evaluated with direct questions about sleep, pain, loneliness, etc.
Statistical analysis
The dependent variable used was the SCD-7 index, the sum of the answers to the questions on SCD. Categorical variables were codified, with higher scores indicating greater burden or severity. According to whether variables were categorical or scalar, associations between the dependent and the independent variables were studied with ANOVA, with an R2 effect size; or contingency tables with a Cramer’s V statistic (interpreted according to the criteria established by Cohen [32]) for effect size and a Pearson correlation coefficient. We created the variable “vascular risk factors,” comprising the dichotomous scores for the variables hypertension, dyslipidemia, and diabetes. Another variable, the “pain index,” constitutes the sum of all pain-related variables (pain [no pain, 0; mild, 1; moderate/intense, 2], low back pain [0/1], neck pain [0/1], migraine/headache [0/1], arthritis/arthrosis [0/1], taking opioids [0/1]) and was scored from 0 to 7. A linear regression analysis was performed to identify predictors, with the R2 and beta statistics used to calculate effect size. Some participants did not respond to any question; these were classified as missing cases and excluded from the analysis. Statistical analysis was conducted using SPSS version 20.0.
Results
Epidemiological data are shown in Table 1.
| Characteristics (n = 1775; age range: 55-98 years) | ||
|---|---|---|
| Variable | n | % |
| Age group | ||
| 55-59 | 386 | 21.7 |
| 60-64 | 370 | 20.8 |
| 65-69 | 315 | 17.7 |
| 70-74 | 251 | 14.1 |
| 75-79 | 189 | 10.6 |
| 80-84 | 153 | 8.6 |
| ≥ 85 | 111 | 6.3 |
| Sex | ||
| Men | 745 | 42 |
| Women | 1030 | 58 |
| Level of schooling | ||
| Primary or below | 438 | 25.5 |
| Secondary | 695 | 39.4 |
| University | 642 | 35.1 |
| Occupational social class | ||
| Group I-II (managerial/university degree) | 631 | 35.7 |
| Group III-IV-V (skilled worker/ administrative) | 647 | 36.6 |
| Group VI-VII (semi/unskilled worker) | 454 | 25.7 |
| No response/ Never been employed | 35 | 2 |
| Living situation: Living alone | ||
| Yes | 350 | 31.3 |
| No | 768 | 68.7 |
Table 1: Sociodemographic characteristics of the sample.
Cognitive complaints were characterized according to current criteria for subjective cognitive decline (Table 2).
| SCD-7 questions | Yes | % | No | % |
|---|---|---|---|---|
| 1) Do you have memory problems? (Total population = 1775) | 290 | 16.3 | 1485 | 83.7 |
| 55-65 age group | 90 | 11.9 | 666 | 88.1 |
| ≥ 65 age group | 200 | 19.6 | 819 | 80.4 |
| 2) Do you have problems with attention or concentration? | 104 | 35.9 | 186 | 64.1 |
| 3) Do these problems worry you? | 178 | 61.4 | 112 | 38.6 |
| 4) Have you seen a doctor about this? | 119 | 41 | 171 | 59 |
| 5) Does this affect your day-to-day life? | 50 | 17.2 | 240 | 82.8 |
| 6) Do you feel that you have worse memory than other people your age? | 46 | 15.9 | 244 | 84.1 |
| 7) How long ago did your memory problems start? | n | % | ||
| <2 years | 85 | 29.3 | ||
| 2-5 years | 103 | 35.5 | ||
| >5 years | 102 | 35.2 | ||
Table 2: Frequency of cognitive complaints, according to the SCD-7 questions.
The mean SCD score was 3.45 points (SD=1.51). Memory complaints were reported by 16.3% of participants, by 19.6% of those older than 65, and by 11.9% of those aged 55-64 years. Mean SCD-7 index score was 3.69 (SD=1.36) in participants with attention problems and 1.75 (SD=1.08) in those without (F=178.50; p<0.001; R2=0.38). Of all respondents with attention problems, 75% were concerned by these (χ2=12.69; p<0.001; V=0.21) and 59.6% had consulted a physician (χ2=23.137; p<0.001; V=0.28). A smaller percentage reported that their everyday lives were affected and that they performed worse than peers of the same age. If we consider the frequency of complaints among the whole sample and the fact that 41% of those with complaints had visited a physician for this reason, then 6.7% of the whole study population had consulted a physician due to memory complaints, with 2.8% reporting an impact on their daily lives. All respondents met at least one of the SCD criteria, with 23.1% (non-cumulative data) meeting two, 24.8% meeting three, 22.1% meeting four, 10.3% meeting five, 9% meeting six, and 3.1% meeting all seven criteria.
Table 3 shows the results of the bivariate analysis of the associations between SCD-7 index score and each of the other variables.
| Variables | Statistic | p-value | Effect size |
|---|---|---|---|
| Sociodemographic variables | |||
| Age | r=-0.05 | 0.445 | - |
| Education (primary, secondary, university) | F=0.69 | 0.502 | - |
| Occupational social class | F=1.94 | 0.054 | - |
| Sex | F=0.04 | 0.846 | - |
| Cognitive performance variables | |||
| Orientation in time | r=0.16 | 0.007 | - |
| Quality of life and mental health variables | |||
| Quality of life (COOP/WONCA questionnaire) | r=0.36 | <0.001 | - |
| Mental Health (GHQ-12) | r=0.41 | <0.001 | - |
| Depression | F=31.87 | <0.001 | R2 =0.10 |
| Anxiety | F=16.72 | <0.001 | R2 =0.06 |
| Taking antidepressants (last two weeks) | F=7.50 | 0.007 | R2 =0.03 |
| Taking sedatives (last two weeks) | F=3.58 | 0.059 | R2 =0.01 |
| Sleep quality | F=4.82 | <0.001 | R2 =0.08 |
| Social support variables | |||
| Living situation: living alone (yes/no) | F=0.11 | 0.743 | - |
| Feeling lonely (yes/no) | F=11.74 | 0.001 | R2 =0.04 |
| Having nobody to turn to for help | F=1.57 | 0.212 | - |
| Acknowledged (by a public agency) disability | F=13.91 | <0.001 | R2 =0.05 |
| Being dependent | F=8.85 | 0.003 | R2 =0.04 |
| Not leaving home because of health problems | F=6.72 | 0.01 | R2 =0.03 |
| Limited activity due to health problems | F=4.84 | 0.009 | R2 =0.03 |
| Disease-related variables | |||
| Perceived health status | F=6.74 | <0.001 | R2 =0.09 |
| Multimorbidity (0-11) | r=0.29 | <0.001 | - |
| Chronic disease (yes/no) | F=11.45 | 0.001 | R2 =0.04 |
| Hospital admission in the last year (yes/no) | F=0.11 | 0.743 | - |
| Pain-related variables | |||
| Pain (no pain/mild/moderate-severe) | F=10.86 | <0.001 | R2 =0.07 |
| Chronic neck pain | F=7.85 | 0.005 | R2 =0.03 |
| Chronic low back pain | F=14.67 | <0.001 | R2 =0.05 |
| Migraine/headache | F=8.32 | 0.004 | R2 =0.03 |
| Arthritis/arthrosis | F=13.02 | <0.001 | R2 =0.04 |
| Taking opioids (last two weeks) | F=14.08 | <0.001 | R2 =0.05 |
| Pain index (range 0-6; 0 = no pain) | r=0.32 | <0.001 | - |
| Disease-specific variables | |||
| Vision problems | F=7.16 | 0.008 | R2 =0.03 |
| Hearing difficulties | F=9.93 | 0.002 | R2 =0.05 |
| Vascular risk factors | F=3.84 | 0.01 | R2 =0.04 |
| High cholesterol level | F=6.61 | 0.011 | R2 =0.02 |
| Heart disease (angina, infarction) | F=0.60 | 0.441 | - |
| Hypertension | F=0.98 | 0.322 | - |
| Asthma | F=0.09 | 0.76 | - |
| Chronic allergy | F=0.84 | 0.361 | - |
| Lung disease | F=0.75 | 0.389 | - |
| Diabetes | F=2.25 | 0.134 | - |
| Gastric or duodenal ulcers | F=2.86 | 0.092 | - |
| Thyroid disease (hypothyroidism, etc) | F=1.70 | 0.194 | - |
| Lifestyle variables | |||
| Alcohol consumption (yes/no) | F=0.78 | 0.378 | - |
| Frequency of alcohol consumption | F=2.25 | 0.108 | - |
| Smoking (yes/no) | F=0.86 | 0.354 | - |
| Physical activity (none/light/moderate/intense) | F=1.13 | 0.339 | - |
| Pet ownership (yes/no) | F=0.40 | 0.527 | - |
| Size of house | F=0.54 | 0.464 | - |
| Economic difficulties | F=1.87 | 0.099 | - |
| Mobile phone use (yes/no) | F=1.91 | 0.154 | - |
| Discomfort due to environmental noise | F=0.74 | 0.392 | - |
| Diet: vegetable intake | F=0.96 | 0.442 | - |
GHQ-12: 12-item General Health Questionnaire
Table 3: Association between SCD-7 index score and other variables.
Orientation in time is significantly associated with SCD-7 index score; the association is stronger in the >65 years age group (r=0.19; p=0.008) than among individuals aged 55-64 (r=0.13; p=0.22). Respondents slept for a mean of 7.8 hours/day (SD=8.80). Hours of sleep showed a nonsignificant correlation with SCD-7 index score (r=0.02; p=0.78); however, loss of sleep due to concerns about cognitive performance did show a significant association (F=26.11; p<0.001; R2=0.08).
Multimorbidity and HRQoL were also associated with SCD-7 index score. The Pearson correlation coefficient for the latter association was r=0.47 (p=0.001). HRQoL was associated with SCD independently of multimorbidity (partial r=–0.26; p<0.0001), depression (F=27.95; p<0.001), anxiety (F=23.23; p<0.001), and pain index score (partial r=0.22; p<0.001). Mean pain index score among participants with depression was 3.54 (SD=2.03), vs. 2.17 (SD=1.83) among those without depression (F=26.13; p<0.0001; R2=0.08); this interaction was not statistically significant (p=0.36). Multimorbidity was also associated with perceived health status (χ2=31.76; p<0.001; V=0.33).
A significant association was found between the variables “feeling lonely” and “living alone” (χ2=72.17; p<0.001; V=0.26), although only the former was associated with SCD-7 index score. Among those who did not live alone, 24.5% reported loneliness, as compared to 49.2% of those participants who did live alone. We analyzed whether this association is conditioned by depression, given the association between depression and loneliness (χ2=80.89; p<0.001; V=0.21): depressive symptoms were recorded in 31.6% of those participants who reported feeling lonely vs. 13.1% of those who did not (χ2=14.53: p<0.001; V=0.22). SCD was independently associated with both variables (F=12.57; R2=0.12; p<0.0001) and the interaction was not statistically significant (p=0.60). Living with 2 or more other people was not associated with any difference in SCD-7 index score (F=0.02; p=0.981).
We studied the interaction between social support variables and the SCD-7 index, finding an interaction between the variables “having nobody to turn to for help” and “being dependent,” with higher scores in individuals meeting both criteria (F=5.44; p=0.001; R2=0.07; R2 for interaction: 0.03; p=0.011). The remaining interactions were not significant. Significant associations were observed between each of the social support variables and depression (p=0.04 for “having nobody to turn to for help” and p<0.001 for the remaining variables), but their interaction with respect to SCD was not significant (p>0.05 for all variables).
Multivariate analysis
A multivariate linear regression study was performed to identify predictors of SCD-7 index score (Table 4). The analysis included all variables showing a significant association in the univariate study and showing at least a small-to-medium effect size. A hierarchical block-based method was used (first block: orientation in time; second block: GHQ-12 score, depression, anxiety, sleep quality, quality of life; third block: loneliness, recognized disability, being dependent; fourth block: perceived health status, pain index, multimorbidity, hearing difficulties, chronic disease). We obtained three significant models; the variables in the third block, which we refer to as social support variables, were not significant. The variables finally identified as predictors were: depression, sleep quality, multimorbidity, and hearing difficulties (F=13.99; p<0.001; R2=0.30). Given its significance in the first model, orientation in time was maintained in the last model. Mental health was significant only in the second block (Table 4).
| Model R2 | Predictors | Non standardized coefficients | Standardized coefficients | t | sig. | |
|---|---|---|---|---|---|---|
| B | Standard error | β | ||||
| R2=0.025 | Temporal Orientation | 0.29 | 0.12 | 0.16 | 2.1 | 0.022 |
| R2=0.25 | Temporal Orientation | 0.19 | 0.11 | 0.11 | 1.75 | 0.082 |
| Mental Health (GHQ-12) | 0.1 | 0.04 | 0.19 | 2.49 | 0.014 | |
| Depression | 0.82 | 0.26 | 0.23 | 3.17 | 0.002 | |
| Sleep Quality | 0.55 | 0.16 | 0.23 | 3.54 | 0.001 | |
| R2=0.30 | Temporal Orientation | 0.16 | 0.11 | 0.09 | 1.51 | 0.132 |
| Mental Health (GHQ-12) | 0.08 | 0.04 | 0.14 | 1.93 | 0.055 | |
| Depression | 0.71 | 0.25 | 0.2 | 2.79 | 0.006 | |
| Sleep Quality | 0.51 | 0.15 | 0.21 | 3.34 | 0.001 | |
| Number of diseases (0-11) | 0.12 | 0.05 | 0.15 | 2.3 | 0.022 | |
| Hearing difficulties | 0.69 | 0.26 | 0.16 | 2.65 | 0.009 | |
Dependent variable: SCD (Subjective Cognitive Decline)
Table 4: Linear regression statistics; Model Summary and Predictors of SCD.
Discussion
We performed an epidemiological study on SCD with a sample taken from a City Health Survey and selected from the city census. No association was identified between SCD and age, sex, level of schooling, occupational social class, or living situation. Similar findings have been reported in other studies: in a population study including 8834 participants, Luck et al. [33] found that neither age, sex, level of education, nor social class was associated with cognitive complaints; likewise, Slavin et al. [8] report that the factors age, sex, and education had very little impact on cognitive complaints. SMCs were less frequent in our sample than in most other epidemiological studies. Although, the 2005 City of Madrid Health Survey [34] found a rate of 32.4%, which is more aligned with the literature; the use of proxies for data collection in 2005 study may have contributed to this. However, with regard to SMCs the specific studies do report an association with sociodemographic variables [35]. The association between SCD and age is particularly noteworthy: according to the studies cited, SCD shows a weaker relationship with age than do SMCs. What might explain this difference in the behavior of SMCs and SCD? One reason may be the fact that the concept of SCD includes other components besides memory (concern, consultation with physicians, time since onset, etc.), which are independent of these sociodemographic factors.
Multivariate analysis
The multivariate analysis aimed to identify predictors of SCD. Orientation in time was included in the first block in the regression analysis, and was significant only in the first model, indicating that this variable is not significant after controlling for the other variables included, given its small effect size. In other words, decreased cognitive performance is present in SCD but is not a powerful factor. It should be noted that rather than being a static condition, SCD changes over time in each individual and cognitive performance might present different levels of impairment depending on when assessment is performed. The remaining variables with the greatest effect were those related to mental health: general mental health (which was not significant in the third block), depression, and sleep quality. Mental health alterations, and especially depression, have a direct negative effect on memory performance [35] and can contribute to increased awareness of an individual’s cognitive mistakes. Several phenomena occur in depression: recall is better if the information or fact is consistent with the person’s mood (negative occurrences are better remembered); in addition, individuals with depression also present “hindsight bias,” whereby neutral and even positive occurrences may take on negative connotations [36]. Depression is one of the factors most consistently associated with SMCs in the literature [37]; the same association is seen with SCD. Another associated factor is sleep: less satisfactory, poor-quality, fragmented sleep with frequent arousals, early morning awakening, and changes in sleep architecture is reported in elderly individuals [38]. Sleep is important in consolidating recent memories into long-term memory; therefore, sleep alterations can contribute directly to reduced memory performance [39].
Other predictors of SCD are hearing difficulties and the number of diseases a person presents. Multimorbidity has been associated both to SMC and to SCD [15]. Our findings show that individuals with SCD present more diseases, and that multimorbidity is associated with poorer perceived health status and poorer quality of life. Furthermore, multimorbidity may have a direct, lasting effect (in the case of cardiovascular risk factors, chronic respiratory failure, treatments, etc.) on brain function, which may affect memory and attention in particular. Some authors have associated vision problems and hearing difficulties with SMCs [40]. According to Lindenberger and Baltes [41], visual and auditory acuity function as indicators of cerebral integrity. Hearing difficulties lead to isolation and a sense of being different from those who are able to communicate with one another. Visual and auditory alterations can have a direct neurophysiological effect on cognitive performance, reducing the capacity to register information. They can also consume attentional resources, as affected people are aware and concerned by their problems with perception and communication, presenting frequent episodes of absent-mindedness. This association confirms the medium-to-high effect size of attention on SCD. Mental health problems, multimorbidity, and sensory difficulties all have a psychological impact, which we could call an “inverse psychological effect” (minimizing one’s own performance and exaggerating deficits). This leads to the perception of one’s body being “sick” and of a general decline, and hence a tendency to exaggerate difficulties and errors (including cognitive errors) and to seek medical attention. This increases the perception of frailty; SCD may therefore be an additional element to be considered within the concept of frailty (cognitive frailty).
Association with pain, social support, and loneliness
Other authors have associated pain with SMCs [42]; in our study, SCD was associated with all types of chronic pain assessed. This association is independent of depression, although depressed individuals did show a higher pain index score at all levels. The association was also found to be independent of depression in other studies [43]. Pain is also linked to attention problems as people focus on pain symptoms: Muñoz et al. [44] report that pain gives rise to SMCs and complaints about concentration. Grisart et al. [45] describe interference between the attentional resources consumed by pain and those dedicated to memory function, this happens above all with the conscious component of remembering, and affects also to memory registration resulting in frequent episodes of forgetfulness. This may be one of the mechanisms explaining the relationship between pain and SCD. Other psychological processes, resembling those discussed in the previous section, are also involved: people reporting pain perceive themselves as “damaged,” describing dissatisfaction and low self-esteem, for which reason they tend to have an exaggerated impression of negative occurrences.
Social support variables (disability, being dependent, not leaving the home/limited activities due to health problems) showed a statistically significant association with SCD-7 index score. Very little research in this field has addressed the lack of social support. Our analysis showed that all of these variables were associated with depression, and that their association with SCD was not mediated by depression (their interaction with depression was not significant). The association may be explained by the inverse psychological effect discussed above: certain deficiencies lead to a feeling of disability and poor self-image, and minor everyday mistakes are exaggerated and take on a special significance.
One special case of a lack of social support is loneliness: lack of the desired or necessary social interaction; it is at once a perception and a feeling. The literature addresses diverse types, causes, and theories of loneliness [46,47]. Loneliness has been associated with poorer cognitive performance [48] and with such other factors as depression [49]. According to Holwerda et al. [50], it is a risk factor for dementia, independently of whether the individual in question is actually socially isolated. In our study, SCD was associated with the feeling of loneliness, but not with social isolation/solitude [51]. In our sample, depression was associated with loneliness; however, the association between loneliness and SCD was not due to depression, as there was no interaction between these variables with respect to SCD. Numerous factors may contribute to the relationship between loneliness and SCD. Physiologically, loneliness is associated with increased activation of the hypothalamic-pituitary-adrenal axis; individuals reporting loneliness display hypervigilance for external threats, particularly social threats [52]. These factors may reduce the available cognitive resources, especially effortful attentional processes, leading to increased attentional bias, which results in absent-mindedness and forgetfulness. People reporting loneliness also present greater levels of anxiety and stress, lower self-esteem, tend to focus more on themselves and compare themselves with others feeling that they have little social value. Consequently they have constant negative thoughts about themselves, feel that their problems and performance are worse than those of their peers, and even make more frequent use of healthcare services and more frequently consult physicians (comparisons against others and medical visits are both components of SCD) [47,52].
Quality of life
According to our findings, HRQoL is associated with SCD, with a medium effect size. Numerous studies address this issue. In their review of SCD and HRQoL, Hill et al. [12] report that the presence, frequency, and severity of SCI are associated with poorer quality of life, independently of the type of study or the sample selected. It is important to establish the causal direction of this association; the majority of authors, including Sohrabi et al. [53] consider SCI to be the cause of poor HRQoL, through depression, anxiety, and personality type. Our results suggest that the association between SCD and HRQoL is independent of anxiety, depression, multimorbidity, and the pain index, although these factors do have some effect, discretely increasing the power of the association. As the study was cross-sectional, we cannot establish the causal direction. However, given the evidence and the data reported by other authors [54], we suspect that the effect is bidirectional. SCD, particularly in people reporting concern, leads to dissatisfaction, poorer self-perceived health status, and fear of becoming dependent and developing cognitive impairment over time [55], which would contribute to poorer quality of life. On the other hand, poorer quality of life may contribute to SCD through the inverse psychological effect, with poorer self-perceived health status due to various factors involved in HRQoL leading to an exaggerated perception of everyday memory lapses and to SCD.
We analyzed whether SCD-7 index score was associated with certain lifestyle factors (smoking, drinking alcohol, exercise), finding no relationship. Neither were the following variables associated with SCD-7 index score: pet ownership (which could influence loneliness), size of home, or economic difficulties.
For older persons who feel and report memory decline, we propose a model of the origin and causation of SCD. Three types of factors can contribute individually or in combination: 1) Perception of a discrete, real cognitive decline caused by aging, various chronic diseases, cardiovascular risk factors, depression, sleep or hearing alterations, reduced external demand (due to retirement), or other causes. 2) Various situations (pain, loneliness, multimorbidity, etc.) consume considerable attentional resources, leading to an increase in everyday memory lapses. 3) Mental health problems, lack of social support, again loneliness and pain, etc. give rise to what we refer to as the inverse psychological effect: these conditions are associated with low self-esteem or low mood, leading to exaggerated awareness of real or perceived cognitive deficits. Our findings are consistent with the literature in that subjective psychological factors have a greater weight in SCD than objective cognitive performance [8]; however, the interaction between the two types of factors is unclear.
Limitations
In our study, all questions on SCD are conditional on the first (“Do you have memory problems?”). This represents a limitation, as all seven questions could have been asked independently; however, this was not the case due to the conditions of the survey. Another limitation is inherent to most studies based on telephone interviews: some individuals decline to participate. Our population study did not use replacement to compensate for this. We may also assume that a considerable percentage of potential respondents with communication and comprehension difficulties opted out; it is likely that this group includes the majority of individuals with cognitive impairment. Considering these issues and the data published on our country in other research [56], the population studied can be considered potentially “normal” (without cognitive impairment) according to the orientation for time domain of the Mini–Mental State Examination [24].
Conclusion
SCD and SMCs are a frequent phenomenon among the older population; in a sample of individuals older than 55 years, a considerable percentage reported cognitive complaints, concern about these, and even medical consultations. However, this phenomenon has multiple causes. SCD is partially associated with poor cognitive performance, but also with such other variables as mental health problems (particularly anxiety and depression), quality of life, pain, multimorbidity, certain diseases and disorders, and loneliness. Early detection of SCD and assessment of its causes are important. Individuals with SCD at risk of AD should be differentiated from those with other associated factors and less risk; the two groups require different management. It is also important to assist older people in proper understanding and evaluation of their memory lapses.
REFERENCES
- Jessen F, Amariglio RE, Van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(6): 844-852.
- Scheef L, Spottke A, Daerr M, Joe A, Striepens N, Kolsch N, et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79(13): 1332-1339.
- Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69(2): 223-229.
- Rabin LA, Smart CM, Amariglio RE. Subjective cognitive decline in preclinical Alzheimer's disease. Annu Rev Clin Psychol. 2017;13: 369-396.
- Nikolai T, Bezdicek O, Markova H, Stepankova H, Michalec J, Kopecek M, et al. Semantic verbal fluency impairment is detectable in patients with subjective cognitive decline. Appl Neuropsychol Adult. 2018;25(5): 448-457.
- Fonseca JA, Ducksbury R, Rodda J, Whitfield T, Nagaraj C, Suresh K, et al. Factors that predict cognitive decline in patients with subjective cognitive impairment. Int Psychogeriatr. 2015;27(10): 1671-1677.
- Koppara A, Wagner M, Lange C, Ernst A, Wiese B, Konig HH, et al. Cognitive performance before and after the onset of subjective cognitive decline in old age. Alzheimers Dement. 2015;1(2): 194-205.
- Slavin MJ, Brodaty H, Kochan NA, Crawford JD, Trollor JN, Draper B, et al. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatr. 2010;18(8): 701-710.
- Brigola AG, Manzini CSS, Oliveira GBS, Ottaviani AC, Sako MP, Carvalho Vale FA. Subjective memory complaints associated with depression and cognitive impairment in the elderly: A systematic review. Dement Neuropsychologia. 2015;9(1): 51-57.
- Zlatar ZZ, Muniz M, Galasko D, Salmon DP. Subjective cognitive decline correlates with depression symptoms and not with concurrent objective cognition in a clinic-based sample of older adults. J Gerontol B-Psychol Sci Soc Sci. 2017;73(7): 1198-1202.
- Hill NL, Mogle J, Wion R, Munoz E, DePasquale N, Yevchak AM, et al. Subjective cognitive impairment and affective symptoms: a systematic review. Gerontologist. 2016;56(6): e109-e127.
- Hill NL, McDermott C, Mogle J, Munoz E, DePasquale N, Wion R, et al. Subjective cognitive impairment and quality of life: a systematic review. Int Psychogeriatr. 2017;29(12): 1965-1977.
- Scholtissen-In de Braek DM, Hurks PMP, van Van Boxtel MPJ, Dijkstra JB, Jolles J. The identification of attention complaints in the general population and their effect on quality of life. J Atten Disord. 2011;15(1): 46-55.
- Caracciolo B, Gatz M, Xu W, Marengoni A, Pedersen NL, Fratiglioni L. Relation of multimorbidity to subjective and objective cognitive impairment: A population-based twin study. J Alzheimers Dis. 2013;36(2): 275-284.
- Montejo P, Montenegro M, López-Higes R, Montejo B. Subjective memory complaints in elderly: Relationship with health status, multimorbidity, medications, and use of services in a population-based study. Int Psychogeriatr. 2016;28(11): 1903-1916.
- Gasquoine PG. Cognitive impairment in common, noncentral nervous system medical conditions of adults and the elderly. J Clin Exp Neuropsychol. 2011;33(4): 486-496.
- Tandetnik C, Farrell MT, Cary MS, Cines S, Emrani S, Karlawish J, et al. Ascertaining subjective cognitive decline: A comparison of approaches and evidence for using an age-anchored reference group. J Alzheimers Dis. 2015;48(s1): S43-S55.
- Abdulrab K, Heun R. Subjective Memory Impairment. A review of its definitions indicates the need for a comprehensive set of standardised and validated criteria. Eur Psychiatry. 2008;23(5): 321-330.
- Rabin LA, Smart CM, Crane PK, Amariglio RE, Berman LM, Boada M, et al. Subjective cognitive decline in older adults: an overview of self-report measures used across 19 international research studies. J Alzheimers Dis. 2015;48(s1): S63-S86.
- Molinuevo JL, Rabin LA, Amariglio R, Buckley R, Dubois B, Ellis KA, et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement. 2017;13(3): 296-311.
- Gifford KA, Liu D, Romano RR, Jones RN, Jefferson AL. Development of a subjective cognitive decline questionnaire using item response theory: A pilot study. Alzheimer's & Dementia. 2015;1(4): 429-439.
- Rami L, Mollica MA, García-Sanchez C, Saldaña J, Sanchez B, Sala I, et al. The subjective cognitive decline questionnaire (SCD-Q): A validation study. J Alzheimers Dis. 2014;41(2): 453-466.
- Snitz BE, Yu L, Crane PK, Chang CCH, Hughes TF, et al. Subjective cognitive complaints of older adults at the population level: An item response theory analysis. Alzheimer Dis Assoc Dis. 2012;26(4): 344-351.
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3): 189-198.
- Sweet JJ, Suchy Y, Leahy B, Abramowitz C, Nowinski CJ. Normative clinical relationships between orientation and memory: Age as an important moderator variable. Clin Neuropsychol. 2010;13(4): 495-508.
- Tractenberg RE, Weinstein M, Weiner MF, Aisen PS, Fuh JL, Goldma N, et al.. Benchmarking A test of temporal orientation with data from American and Taiwanese persons with Alzheimer’s disease and American normal elderly. Neuroepidemiology. 2005;24(1): 110-116.
- Goldberg DP. The detection of psychiatric illness by questionnaire. Maudsley Monograph, 21, 1972.
- Sánchez-López MP, Dresch V. Cuestionario de Salud General-12 ítems. Psicothema. 2008;20(4): 839-843.
- Greenfield S. The use of functional status assessment within the framework of the International Classification of Primary care. In: Lipkin M (ed) Wonca Classification Committee. Functional status measurement in primary care. Springer-Verlag, New York, USA, 1990;pp: 28-45.
- Pedrero-Pérez EJ, Díaz-Olalla JM. COOP/WONCA: Reliability and validity of the test administered by telephone. Aten Prim. 2016;48(1): 25-32.
- Comijs HC, Deeg DJ, Dik MG, Twisk JW, Jonker C. Memory complaints: The association with psycho-affective and health problems and the role of personality characteristics. A 6-year follow-up study. J Affect Disord. 2002;72(2): 157-165.
- Cohen J. The concept of power Analysis. In: Statistical power analysis for the behavioral sciences (2nd edn) Lawrence Erlbaum Associates, Hilldale, New Jersey, USA, 1988;pp: 1.1-1.7.
- Luck T, Roehr S, Rodriguez FS, Schroeter ML, Witte AV, Hinz A, et al. Memory-related subjective cognitive symptoms in the adult population: Prevalence and associated factors–results of the LIFE-Adult-Study. BMC Psychol. 2018;6(1): 23.
- Montejo P, Montenegro M, Fernández MA, Maestú F. Subjective memory complaints in the elderly: Prevalence and influence of temporal orientation, depression and quality of life in a population based study in the city of Madrid. Aging Ment Health. 2011;15(1): 85-96.
- Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population‐based studies. Int J Geriatr Psychiatr. 2000;15(11): 983-991.
- Groß J, Blank H, Bayen UJ. Hindsight bias in depression. Clin Psychol Sci. 2017;5(5): 771-788.
- Balash Y, Mordechovich M, Shabtai H, Giladi N, Gurevich T, Korczyn AD. Subjective memory complaints in elders: depression, anxiety, or cognitive decline? Acta Neurol Scand. 2013;127(5): 344-350.
- Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: Diagnosis and management. J Gen Fam Med. 2017;18(2): 61-71.
- Darien IL. American Academy of Sleep Medicine. International classification of sleep disorders, (3rd edn) 2014.
- Cutler SJ, Grams AE. Correlates of self-reported everyday memory problems. J Gerontol. 1988;43(3): S82-90.
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9(3): 339-355.
- Westoby CJ, Mallen CD, Thomas E. Cognitive complaints in a general population of older adults: prevalence, association with pain and the influence of concurrent affective disorders. Eur J Pain. 2009;13(9): 970-976.
- Schnurr RF, MacDonald MR. Memory complaints in chronic pain. Clin J Pain. 1995;11(2): 103-111.
- Muñoz M, Esteve R. Reports of memory functioning by patients with chronic pain. Clin J Pain. 2005;21(4): 287-291.
- Grisart JM, Van der Linden M. Conscious and automatic uses of memory in chronic pain patients. Pain. 2001;94(3): 305-313.
- Coyle CE, Dugan E. Social Isolation, Loneliness and Health among Older Adults. J Aging Health. 2012;24(8): 1346-1363.
- Heinrich LM, Gullone E. The clinical significance of loneliness: A literature review. Clin Psychol Rev. 2006;26(6): 695-718.
- Boss L, Kang DH, Branson S. Loneliness and cognitive function in the older adult: a systematic review. Int Psychogeriatr. 2015;27(4): 541-553.
- Cacioppo JT, Hawkley LC, Thisted RA. Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychol Aging. 2010;25(2): 453-463.
- Holwerda TJ, Deeg DJ, Beekman AT, van Tilburg TG, Stek ML, Jonker C, et al. Feelings of loneliness, but not social isolation, predict dementia onset: results from the Amsterdam Study of the Elderly (AMSTEL). J Neurol Neurosurg Psychiatry. 2014;85(2): 135-142.
- Petersen J, Kaye J, Jacobs PG, Quinones A, Dodge H, Arnold A, et al. Longitudinal Relationship Between Loneliness and Social Isolation in Older Adults: Results From the Cardiovascular Health Study. J Aging Health. 2016;28(5): 775-795.
- Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends cogn sci. 2009;13(10): 447-454.
- Sohrabi HR, Bates KA, Rodrigues M, Taddei K, Martins G, Laws SM, et al. The relationship between memory complaints, perceived quality of life and mental health in apolipoprotein ε4 carriers and non-carriers. J Alzheimers Dis. 2009;17(1): 69-79.
- Verhaeghen P, Geraerts N, Marcoen A. Memory complaints, coping, and well-being in old age: A systemic approach. Gerontologist. 2000;40(5): 540-548.
- Roehr S, Luck T, Pabst A, Bickel H, König HH, Luhmann D, et al. Subjective cognitive decline is longitudinally associated with lower health-related quality of life. Int Psychogeriatr. 2017;29(12): 1939-1950.
- Lobo A, Saz P, Marcos G, Día JL, de la Cámara C, Ventura T, et al. Revalidación y normalización del Mini-Examen Cognoscitivo (primera versión en castellano del Mini-Mental Status Examination) en la población general geriátrica. Med Clin (Barc). 1999;112(20): 767-774.
Citation: Montejo P, Prada D, Pedrero-Perez E, Montenegro-Pena M (2020) Subjective Cognitive Decline: Mental Health, Loneliness, Pain and Quality Of Life; Poblational Study. J Aging Sci. 8:218. DOI:10.35248/2329-8847.20.08.218.
Copyright: © 2020 Carrasco PM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.