PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2020) Volume 0, Issue 0
Review on Recent Advance of Vaccine Adjuvants
Getu Ayele*Received: 28-Sep-2020 Published: 20-Oct-2020, DOI: 10.35248/2157-7560.20.S5:003
Abstract
Vaccines are the most feasible and cost-effective strategy for protecting, controlling and reducing infectious disease in both animal species and humans. Despite, the significant use of vaccines, formulation of effective and safe vaccine is needed to afford sufficient protection through usage of appropriate adjuvants. Adjuvants are chemicals, proteins or derivatives of microbial formulated with vaccines to enhance innate and adaptive immune response to vaccine antigens by a variety of mechanisms. The term adjuvant comes from Latin word “adjuvare” which means to help and discovered in early 1920s. Many molecules have been considered as an adjuvant including aluminium salts (alum), oil emulsions, saponins, ISCOMs, liposomes, VLPs, cytokines, combined adjuvants and derivatives of bacteria are among in use and explored vaccine adjuvants. Mainly they have been classified according to their mechanism of action as: delivery system and immunostimulatory adjuvants. The mode of action of these compounds is different. Their action is not yet fully understood due to the complexity of the immune response, but general mechanism has been explained. Recent advances of vaccine adjuvants reveal their ability to activate innate immune system by targeting receptors (PRR) expressed on immune cells. Therefore, discovering new adjuvant that are essential components of vaccine formulations helps the development of more potent vaccine, which induce strong immune response. Better understanding of new adjuvant also improves the future design of effective vaccine against infectious pathogens. This review, provide an overview on current knowledge about the effect of adjuvants, general mechanisms and characteristics of recent vaccine adjuvants.
Keywords
Vaccine; Adjuvant; Innate; Adaptive; Alum; ISCOMs; VLPs; Liposomes; Cytokines; Delivery system; Immunostimulatory; PRR; Pathogen
Introduction
Development of vaccines against infectious diseases is one of the most significant accomplishments in the history of humankind [1]. Live attenuated and inactivated (killed) type of vaccines is currently the most used vaccines both in humans and animals. Attenuated vaccine consists live bacteria or virus causes mild, asymptomatic infection in immunosuppressed individual or whole population but induce strong humoral and cell-mediated immunity similar to natural infection. Consequently, vaccines developed from liveattenuated pathogens can elicit robust protective immune responses because of having naturally existing adjuvants. In contrast, killed and recombinant vaccines are less immunogenic; they require additional components to induce adequate protective immunity [2,3].
Vaccination is one of the most efficient and cost-effective strategy of preventing diseases caused by variety of potential pathogens. Regardless of broad use of vaccines, still development of improved and vaccine with less adverse effect is required to confer protection against both emerging and re-emerging disease. This Sufficient protection can be accomplished by generating strong and effective immune response to the administered antigen often necessitates the addition of another substance termed as adjuvant [4]. Adjuvants are compounds added to vaccine formulations to enhance the immunogenicity of less immunogenic antigens [5].
The mechanisms of action of most adjuvants remain undefined because of the complexity of the immune response. However, concern in vaccine adjuvant has been growing rapidly for better understanding of their action in stimulating immune response. Recent advances in immunobiological research have revealed several mechanisms by which adjuvants act, which includes depot effect or slow release of antigen at injection site, recruiting immune cells at the site of injection, facilitating antigen uptake and by activation of Antigen Presenting Cells (APCs) [6]. Moreover, adjuvants have been developed in order to increase vaccine potency, reduce the dose of antigen desired to elicit immune response, improve speed of immunity as well as reducing the number of immunization or boosting frequency required to attain high level of immunity.
Next generation vaccines are going to mainly encompass recombinant subunit. To provide considerable protection of animals and humans against infectious diseases, subunit vaccines necessitate optimal adjuvants and delivery systems. As a result, there is a vital need of improved adjuvants for the development of new vaccines [7]. Different substances as vaccine adjuvant have been explored with their effect through different mechanisms of action in immune system, including mineral salts (aluminum salts), Oil emulsions, Micro particles, Saponins, Cytokines, microbial products and combined adjuvants(b). Adjuvants have various modes of action and should be selected for use based on the type of immune response desired for a particular vaccine. Better understanding of recent adjuvants will help the development of new adjuvant formulations and facilitate rational design of vaccines against infectious diseases. Nevertheless, many adjuvants have been tested in advanced stages of development, and much of the early work was based on discovery and development of producing safe and effective products of vaccine adjuvants [8]. Therefore, the aims of this seminar paper are: to review the recent advance of vaccine adjuvants, to review general characteristics of different adjuvants and to give insight on mechanisms of action of adjuvants.
Literature Review
Vaccine
Vaccines play an important role in controlling and preventing both animals and humans from infectious disease [9]. Vaccination is used in maintaining animal health and to improve overall production, controlling and reducing zoonotic diseases such as anthrax, brucellosis, rabies, influenza and also control of emerging as well as exotic diseases of animals and human. Furthermore, vaccination strategy plays an essential role to reduce cost of treatment [10].
The most effective vaccines currently in use are attenuated and inactivated forms of infectious pathogens or their products. However, both types of vaccines have their own advantage and disadvantage related to safety, stability, and efficacy in inducing immune response. The goal of these vaccines is to generate a pathogen-specific immune response providing long-lasting protection against diseases. A live-attenuated vaccine which consists of live organisms can induce strong humoral and Cell-Mediated Immunity (CMI). Whereas, inactivated vaccine types are safer than live- attenuated and principally induce humoral immunity, but little or no cell-mediated immunity. Therefore, an addition of adjuvant to vaccine is essential to confer strong protective immunity [4].
The discovery of adjuvants
In the early time there were many attempts by investigators to increase important immune response. They have tried to find useful additives that can enhance immune responses when added to vaccines. Thus, these immune enhancing substances are known as adjuvants [11]. The term adjuvant is derived from the Latin word adjurer, which means to help or to enhance [4]. Adjuvants are substances formulated with vaccine of interest to improve immunogenicity of antigens that have insufficient immunostimulatory. They have been used in vaccines for more than 90 years. Aluminum and oil-in-water emulsions were the first adjuvants used widely and help in the understanding of how immune system interacts with pathogens, role of adjuvants and improved modern vaccine formulation to achieve desired clinical benefit [1].
Reviews historical background of adjuvant development from the early century by classifying into four (4) periods:
• 1920-1940 development adjuvants of toxoid vaccines.
• 1940-1970 extended use of aluminum and oil adjuvants.
• 1970-1990 synthetic adjuvants and second-generation delivery–depot systems were developed.
• After 1990 development of receptor-associated adjuvants that activate innate immune system [10].
In the mid of 1920s, Gaston Ramon French veterinarian injects horse with diphtheria toxoid and observe abscess developed at the site of injection. Consequently, he observed higher yield of antidiphtheria antibody from abscessed animals than animals without abscess. Based on his observation he reports that, abscess formed as a result of foreign substances together with toxoid also induces increased amount of diphtheria antitoxin in horses, which confirm the prediction of substances capable to produce local inflammation at the site of injection also able to enhance production of antisera [5]. About 1926, Alexander Glenny and his colleagues from London revealed the effects of aluminum salt in immune response. In 1932, aluminum was used for the first time in human vaccines and becomes the only adjuvant used in licensed vaccines for nearly 70 years. Even though its use is widespread and continuous its immune mechanism of action remains less understood. Aluminum adjuvants were mainly function to raise or increase production of antibody and consequently suitable for vaccines directing killing of pathogens via antibodies [4].
Water-in-oil emulsion is the other early developed vaccine adjuvant by Freund in the mid of 1930s, containing killed mycobacteria as extra immune-activator called Freund’s Complete Adjuvant (FCA). FCA is characterized as the most effective and very react genic adjuvant. Adjuvant without mycobacteria, known as Freund’s Incomplete Adjuvant (FIA), also developed by Freund and employed in licensed influenza vaccine in United Kingdom. Later on, in 1956, adjuvant activity of endotoxins of gram-negative bacteria discovered by Arthur Johnson and in 1974 muramyl dipeptide from mycobacteria in FCA revealed as smallest active component of adjuvant [5].
Roles of adjuvants
Immunological adjuvants, initially described by Gaston Ramon are substances used in mixture with a desired antigen to increase effective immune response than the antigen alone [12]. These substances rise immune response in various ways: increasing immunogenicity of less or weak immunogenic antigens, inducing long-lasting immunity, activating of Cell Mediated Immunity (CMI), [13,4] triggering mucosal immunity, enhance immunity of immune-suppressed individuals, reducing dose of antigen, subsequently reduce costs and help to overcome competition of antigens in combined vaccine (Figure 1).
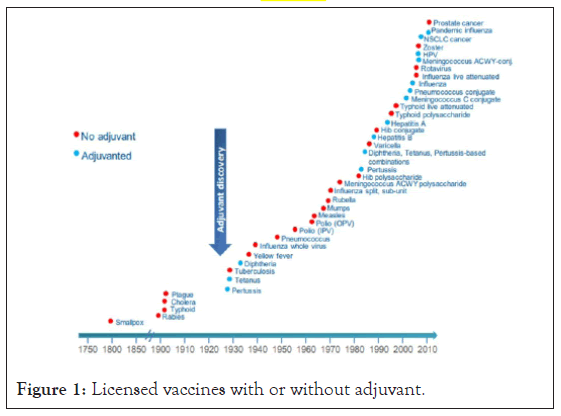
Figure 1: Licensed vaccines with or without adjuvant.
Classification of adjuvants
Classification of vaccine adjuvants can be based on their source (natural, synthetic or endogenous), mechanism of action, and physical or chemical properties. Based on their mechanisms of action, they can be divided into delivery system (particulate) adjuvants and immune stimulatory or non-particulate adjuvants. As the name specifies the first classification of adjuvant, function by delivering antigen to immune cells (antigen presenting cells particularly, dendritic cells). Delivery system adjuvants are designed to activate innate immune response through creating local proinflammatory response that leads to recruiting of various cells to the site. The later, immune stimulatory adjuvants are designed to activate innate immune responses by means of Pattern-Recognition Receptors (PRRs) which consist various classes of receptors includes Toll-like receptors, Nucleotide-binding Oligomerization Domain- (NOD-) like receptors, and the Retinoic Acid-inducible Gene-I (RIG-I-) like receptors, all are expressed on cells of immune systems [14]. Finally, engagement of Pathogen-Associated Molecular Patterns (PAMPs) initiates activation of innate cells that can eventually transfer to other tissues and results in production of cytokines and chemokine (Figure 2).
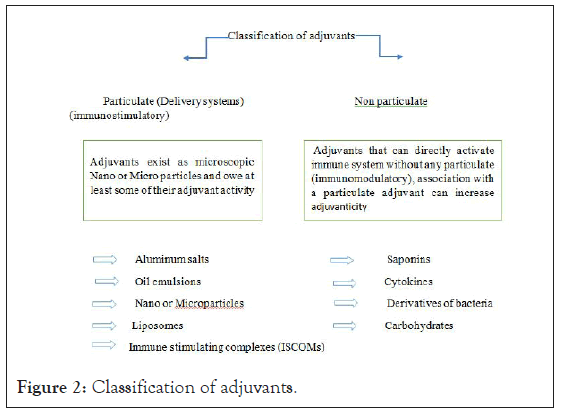
Figure 2: Classification of adjuvants.
Mechanisms of action of adjuvants
Better understanding of mode of action of adjuvants or immunological mechanisms is critical in designing new vaccine adjuvants that enhance effective immune responses towards pathogen-specific and appropriate memory. It also assists in evaluation of adjuvant safety at developmental stage [15]. Based on their mechanism of action they have been divided into delivery system and immuno-stimulatory adjuvants. In the early time, delivery system was considered only to act as depot adjuvant while immune stimulatory induce innate immune response, but now days there is confirmation that some of delivery system can also activate cells of innate immune system [13].
Vaccine adjuvants are commonly used in billions dose of humans and animals’ vaccine. However, their mechanisms of action are not well understood [13]. However, several mechanism of adjuvant action has been revealed. Some adjuvants can act by slow release or trap of the antigen at the site of injection and present a sustained supply to local APCs (Depot effect). This effect helps to reduce removal or degradation of the antigen by immune cells (i.e. liver). Recruitment of cells at the site of injection, regulation of cytokines and chemokine’s, enhancement of expression of Major Histocompatibility Complex (MHC) class II and co-stimulatory molecules, and induction of inflammatory cascades are mechanisms employed by adjuvants to invoke immune response [16].
Generally, all the mechanisms include stimulation of APC directly or indirectly, mainly Dendritic Cells (DC). DC activates innate and adaptive immune system by processing the antigens and presenting to specific T-cells. The trapped antigen taken up through the phagocytosis or pinocytosis by DC and then detected by Pattern- Recognition Receptors (PRR). During stimulation of PRR, several soluble inflammatory mediators such as cytokines and type 1 interferon (IFN-1) are released by naive DC as part of innate immunity. Additionally, adaptive immune response also stimulated by activated DC, via processing and presenting the antigens to specific T-cells (CD4+). MHC II and co- stimulatory molecules are also activated by DC to help interactions between DC and CD4+ T cells (Figure 3). Increased CD4+ is stimulated as a result of this immunological cascade, but this cascade is inadequate for CD8+ T cell stimulation, which is important for efficacy of vaccine against cancer and intracellular pathogens. Therefore, novel adjuvants are targeted to receptors of APCs expressed on DCs to activate the innate immune system. PRRs include the TLRs, CLRs, and NLRs. presenting antigens to APCs with adjuvant permits induction of immunity against tumors or antivirals [17,18].
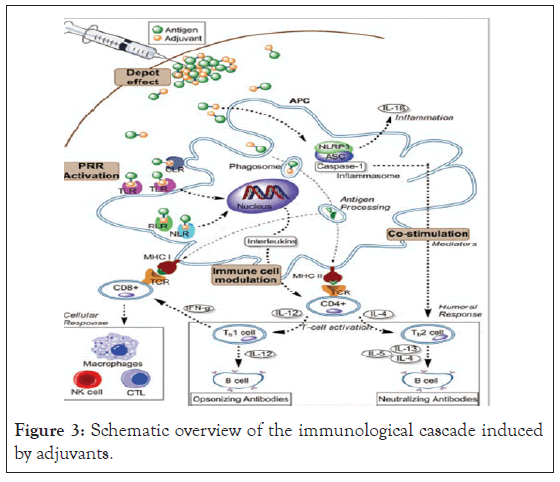
Figure 3: Schematic overview of the immunological cascade induced by adjuvants.
Major Types of Adjuvants
In general, based on principal mechanisms of action, adjuvants can be classified into delivery systems (particulate) and immunostimulatory adjuvants (non-particulate. Vaccine delivery systems includes emulsions, micro particles, Immune-Stimulating Complexes (ISCOMs) and liposomes, function to deliver antigen to APCs. Immunostimulatory adjuvants are primarily resulting from pathogens; represent Pathogen Associated Molecular Patterns (PAMP), function to activate innate immune cells. Recently, there are also combined adjuvant from delivery systems and immunostimulatory agents, to deliver immunostimulatory into APCs more effectively [13].
Non-particulate adjuvants
Non-particulate also called immunostimulatory adjuvants are substance that can act directly to stimulate immune systems without depending on particles, but the combination with particulate adjuvants increases their effectiveness. Non particulate adjuvants include aluminum, oil emulsions, saponins, cytokines, molecules derived from bacteria and Carbo Hydrates (CH).
Aluminum based adjuvant: Aluminum Salts Include Aluminum Hydroxide [Al(OH)3] and Aluminum Phosphate (AlPO4), often referred as alum, has been principally the most used adjuvants in vaccine for human and animals for over 80 years [19]. Aluminum Hydroxide [Al(OH)3] is the most widely used chemical as adjuvant [20]. Aluminum salts as an adjuvant first reported by Alexander Glenny and his colleagues in 1926. In his report aluminum potassium sulfate with toxoid injected into laboratory animals induces higher antibody production [17]. Accordingly it is the gold standard against all new adjuvants can be compared; adjuvants proposed to induce a protective immunity for new candidate vaccine should be first compared with alum before further experiments. Despite, the wide use of this adjuvant its mechanism of action is still not fully clear [21].
Alum-based adjuvants are largely considered to induce strong Th2, but little or no Th1 immune response. However, recent studies demonstrate that aluminum hydroxide-based adjuvants can provoke both Th1 as well as Th2 immune responses depending on the route of vaccination [20]. Since, Th1 cytotoxic responses are essential against tumor and virus-infected cells to activate it other stimuli like Toll-like receptor response (TLR)are required. Trigger TLR4, TLR7, and TLR9 can activate cytokines and IFN-1 that stimulate immune system to activate Th1 responses. Some study trials of TLRs shows that, TLR ligands can be effective and safe adjuvants for vaccines licensed in Europe and USA [22].
Mechanism of action of aluminum hydroxide-based adjuvants comprises the depot effect, pro-phagocytic effect, and activation of the Nucleotide Binding Oligomerization like receptor protein 3(NLRP3) inflammasome activation. These all stimulate innate, adaptive immune responses and complement system [18]. Aluminum-based adjuvants (mixture of aluminum and antigen) are known by having depot effect, slowing the rate of release of antigen to immune system by being adsorbed or attached to the surface of adjuvants containing aluminum and finally leads to retention of antigens at injection site. However, recent studies have clearly confirmed that depot effect is not needed for alum adjuvant city; excision demonstration of injection site did not inhibit development of immune response [4]. The depot effect is important in long-lasting and effective activation of immune response [20].
The other mechanism of aluminum based adjuvant is activation of Nucleotide-binding Oligomerization Domain (NOD) like receptor protein 3 (NLRP3) inflammasome pathway and IL1β production, which could explain its ability to generate local inflammation, to recruit APCs, activate Dendritic Cell (DC) maturation, stimulation of T-cell activation and differentiation. Oligomerization of NLRP3 undergoes through Caspase Activation and Recruitment Domain (CARD). Activation of (NLRP3) inflammasome pathway has been demonstrated by using ovalbumin antigen with alum in NLRP3 deficient mice and wild-type mice (with NLRP3), the result of this study shows that antibody production in NLRP3 deficient mice is significantly lower than wild-type mice. This study indicates the role of alum based adjuvant in innate and adaptive immune response through activation of NLRP3 inflammasome pathway [23,18,17].
Investigators through in vitro experiments have tried cells targeted by aluminum hydroxide-based adjuvants to trigger immune response. Al(OH)3 act on macrophages, facilitates their differentiation into dendritic cells and increase delivery of antigens by macrophages rather than activating DC. It can also facilitate recruitment of inflammatory monocytes into injection site and their differentiation into DCs. (Figure 4). Recruited inflammatory monocytes express higher levels of Major Histocompatibility Complex class II (MHC II) because of improved capacity to adsorb antigens. Therefore, DCs (antigen carrying) differentiated from inflammatory monocytes migrate to lymph nodes and then encourage proliferation of T cell [24].
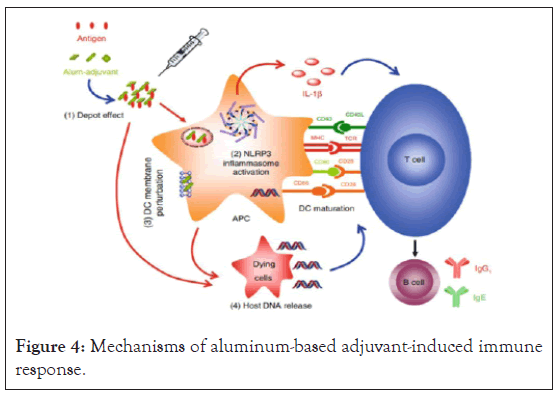
Figure 4: Mechanisms of aluminum-based adjuvant-induced immune response.
Oil emulsion adjuvants: The major emulsions used in vaccine, with one substance dispersed into the others are: oil-in-water (O/W) and water-in-oil (W/O) emulsions. Jules Freund introduces water-in-oil emulsions such as FIA adjuvants in 1930 for the first time and it is widely used adjuvant. Like aluminum, the water-inoil emulsions adjuvant function as delivery systems by formation of depot effect that trap antigen at injection site and by slowing release of antigen in order to induce long-lasting immune response [12]. In addition, water in-oil adjuvants can protect antigens from degradation by peptidase enzyme [18]. Emulsions adjuvants can induce production of immunostimulatory cytokines and chemokines and activate the recruitment of Dendritic Cells (DCs), monocytes, which differentiate latter to DCs [25].
Water in oil emulsions adjuvant contains micro droplets of an aqueous phase in oil, stabilized by a surfactant. They act by slow releasing of the antigen and provide long term immunity. However, reaction at the site of injection and viscosity are common with water in oil emulsion due to these using in vaccine of human and companion animals are not recommended, but they can use in some vaccine of ruminant, poultry and in research animals. In contrast to W/O adjuvant, O/W emulsions such as MF59 have oil in water, stabilized by surfactants. MF59 emulsion is widely used emulsion adjuvant which able to stimulate both cellular (Th1) and humoral (Th2) immune responses [26]. They release antigen quickly and induce short-term immunity. Relative to w/o emulsions o/w emulsion is less viscous and less activate inflammation [16].
Bacteria-derived adjuvant: Components of bacteria have strong capacity to stimulate immune system, thus substances derived from bacteria can act as vaccine adjuvants. Cell wall structure such as lipopolysaccharide of gram negative, muramyl dipeptide of mycobacteria bacteria administered with less immunogenic antigens enhances immune response. Mycobacterium species, Corynebacterium species, Bordetella pertussis and Neisseria meningitides are species of bacteria used as source of adjuvants [27] (Figure 5).
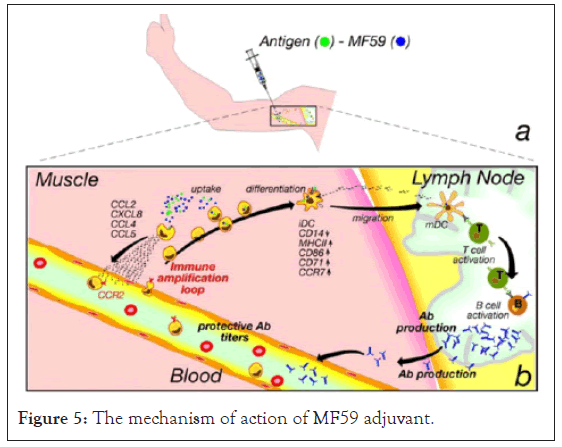
Figure 5: The mechanism of action of MF59 adjuvant.
Lipopolysaccharide: The outer membrane of gram negative bacteria encompasses surface components called Lipopolysaccharides (LPS), which composed of polysaccharide (hydrophilic) and phospholipid or lipid A. Lipid A is the antigenic component of LPS responsible for immune response via recognition of TLR (TLR4) and directs DC toward Th1 immunity. However, lipid A as an adjuvant is often related with high toxicity. In low acid conditions, lipid A can be hydrolyzed to obtain Monophosphoryl Lipid a (MPL), a compound which retains the adjuvant activity of lipid A with reduced toxicity. Hence, to overcome toxicity of Lipid A, elimination of the phosphate group and 3-O-deacylation of lipid A generates Monophosphoryl Lipid A (MPL) with retaining its immunostimulatory (adjuvanticity) property [18].
Muramyl dipeptide: Historically, heat inactivated mycobacteria was used as adjuvants, for example, Freund complete adjuvant that contains mycobacteria [16]. The cell wall of mycobacteria constitutes immune reactive proteins called Muramyl Dipeptide (MDP) consisting N-acetyl muramic acid. Muramyl dipeptide is the active component capable of stimulating immune response through activating production of proinflammatory cytokines (TNF α, IL-1, IL-6, and IL-8), secretion of reactive products, Th2 and enhancing cytotoxicity. Adverse effect has been observed in MDPbased adjuvants because of this, muramyl dipeptide derivatives able to induce Th2 and Th1 have been developed in order to reduce adverse effects [16,14].
CpG oligodeoxynucleotides: Many experiments carried out on effective vaccine adjuvants were targeted various components of innate immune signaling pathways termed as Pathogen Recognition Receptors (PPR), particularly TLRs [24]. PPRs are first line of defense against infectious microbes by recognizing PAMPs found only in microbes but not in the host. Among PAMPs of pathogens, cytosine phosphate guanidine (CpG) motifs are well known in bacteria and virus [17]. CpG oligodeoxynucleotides (ODNs) are short synthetic DNA molecules consists of 18-25 base sequence and methylated CpG motifs. CpG ODNs, type of TLR 9 agonists, can activate both innate, antigen specific immunity and proinflammatory cytokines TNF-α, IL-1, IL-6 and IFN-γ [14,16,10]. The use of CpG oligonucleotides have been tested in animals and proven as it is effective in large animals [28]. Regarding the safety of CpG based adjuvants remains to be determined. Some studies conducted on the dose of these adjuvants demonstrates that low doses become safe and administration of repeated high dose to mice resulted in splenomegaly, release of cytokine and fatal shock. CpG oligonucleotides incorporated into the sequence of DNA vaccines or antigens may minimize these adverse effects [16].
Although, carbohydrate-based adjuvants such as glucans, fructans, mannans, chitin/chitosan and other carbohydrate compounds derivatives of Mycobacterium species (muramyl dipeptide/MDP, trehalose-6–6-dimycolate, LPS and saponin compounds can be function as vaccine adjuvants [27].
Particulate-based adjuvants
Nanoparticles and microparticles are the common form of Particle-based adjuvants that have been explored widely in vaccine formulation [28]. Nanoparticles and micro particles are very small solid particles composed of biodegradable polymers and copolymers and differ from each other in their size, 10-1000 nm and 1-100 μm respectively. Different natural and synthetic polymers have been developed and tested; the most common are Polylactic Acid (PLA), polysterene, chitosan, polyethylene glycol and their copolymers [16]. Microparticles function in delivering antigens of designed vaccine directly to APCs, then as they are phagocytic cells they can uptake particulate antigen provided by these microparticles. Furthermore, particulate-based vaccine can be advantageous in delivering vaccine into mucosal surfaces comprise oral and nasal routes of delivery. Microparticle adjuvants cannot typically act as immunomodulatory, rather they allow incorporation of immunomodulatory or antigens to increase their effect in immune response. Incorporated antigens to particles can be protected from several factors that alter their effectiveness, such as acidic PH, bile salts and enzymatic activities [28].
Saponins: Saponins are natural complex chemical adjuvant widely distributed and extracted from plants, mostly from Quillaja saponaria Molina [29,30]. Saponin-based adjuvants have been used commonly in veterinary vaccines to increase both antibody mediated and cell mediated immune response. Despite, the wide use and immune-stimulating ability of saponin, mechanism of action remains not totally understood [31].
Quil-A, isolated from Quillaja saponaria Molina is the most widely used saponin based adjuvants. This adjuvants have capacity to induce strong Th1 immune response and Cytotoxic T-Lymphocytes (CTLs) which makes them perfect to use in subunit vaccines and vaccines desired for intracellular pathogens [32]. It can induce production of cytokines such as interleukins and interferon’s that might mediate their immunostimulant effects [33]. Quil A has been used broadly in veterinary medicine in vaccines of cattle, pigs, horses, dog and vaccines which contains equine influenza virus, canine parvovirus and FeLV vaccines [16]. The use of Quil A adjuvant in human is limited because of its toxicity and reactogenic properties [30].
Immune stimulating complexes: Immune Stimulating Complexes (ISCOMs) are complexes that contain saponins, cholesterol, and phospholipids. Quillaja saponins associated with lipids and antigens to form ISCOMs contain adjuvant and antigen in the same particle that have distinct properties of adjuvant [33]. A substance derived from saponins and termed as Quil-A is the basis for formation of immuno-stimulating complexes [34]. Currently two types of ISCOMs particles have been described: ISCOM the usual Complexes, which contains cholesterol, saponin, phospholipid and antigen. The second is ISCOM matrix, different from the former in the absence of antigen. ISCOM matrix is based on ISCOM that is mixed with antigen prior to delivery and in case of ISCOM the antigen is fused during the construction process [18]. Structurally both ISCOM and ISCOM matrix are similar; they are cage-like particles of about 40 nm in diameter spherical, and hollow rigid. The interaction between saponin and cholesterol molecules forms stable ring ISCOM or ISCOM matrix in aqueous solutions [35,36].
ISCOMs have capacity to stimulate strong immune response via induction of Th1, Th2 and cytotoxic lymphocytes, including cytokine secretion. Immune stimulating complexes are complexes of partially purified saponins from Quil-A used in vaccines for humans and animals. They were found to be efficient in different animals and man, for instance ISCOMs based adjuvant of an equine influenza vaccine is currently available on the market for horse in Europe. Toxic effects and adverse reaction have also observed in some species of animals, most likely due to a dose effect [36]. The complex ISCOM matrix formed from saponin, phospholipid and cholesterol has been developed to reduce hemolytic activity of saponins, increase stability and interaction with variety of antigens (“Saponins as immunoadjuvant agent: A review,” 2014).
Liposomes: Liposomes are natural or synthetic vesicles comprise lipid bilayer that capture antigen in their membrane or aqueous core and function as both vaccine delivery and immuno potentiators. Hence the antigenic determinant can be encapsulated in the membrane, incorporated in lipid bilayer or being attached to the outer surface of liposomes [18]. Liposomes –based adjuvants are versatile, have been used widely in vaccine experiments and their effectiveness can be influenced by layer of lipid composition, the way antigen and adjuvant interacts or electric charge, size and method of formulation [27]. Liposomes and other liposome-derived nanovesicles including archaeosomes and virosomes also become important delivery systems, thus the concern for liposome-based vaccines has significantly increased [37]. The use of liposomes as vaccine adjuvants was discovered early in 1974 by Gregoriadis and his colleagues, where mice vaccinated using Diphtheria Toxoid (DT) administered with liposomes- based adjuvants revealed that increase in antibody titers than mice vaccinated without liposomesbased adjuvant [38]. After these studies, the role of liposomes in vaccine formulation as adjuvants has been widely discovered and tested. Liposome-antigen complexes injected into mice induced strong cell mediated response when compared with alum-antigen complexes [39]. Moreover, describes that, liposomes formulated with peptide epitopes resulting from Hepatitis C Vaccine (HCV) and Severe Acute Respiratory Syndrome (SARS) coronavirus (SARS-CoV) that adsorbed the surface of liposome, induces significant Cytotoxic T-Lymphocytes (CTL) [18].
Although their mechanism of action is not completely understood, but they interact with APCs such as microphages then increase the presentation of antigen and immunostimulators to the APCs as well as increase retaining of antigen at the site of injection. The cationic properties of liposomes and the presence of anionic on membrane of APCs allows interaction of liposomes with APCs, essential for antigen delivery since they enhance uptake and presentation of antigen by APCs [39].
Virus Like-Particles and Virosomes: Virus-Like Particles (VLP) are molecules which resembles viruses composed of different recombinant structural proteins of viruses including viral capsid or envelope that mimic complete size, shape and molecule organization with properties of self-assembly. VLPs are significantly immunogenic because of having self-adjuvant properties or possess their own adjuvant activity [14]. Since, VLPs contain no genetic materials (DNA/RNA) they are non-infectious, non-replicative and safe. They have the ideal size of 20-100 nm in diameter that helps them to be taken up by cells of immune system such as Dendritic Cells (DC) and microphages. The VLPs structures can be enveloped or non-enveloped according to the wild type/ parental virus. Enveloped VLPs, composed of host cell membrane as an envelope combined with antigen of interest, whereas nonenveloped are formed only from component of the virus with having the capability to self-assembly. Toll-like receptors (TLRs) agonists, a substance which able to initiates immune response when bound with receptor can also be components integrated into VLPs [40].
Virus Like-Particles can induce both Cell Mediated (CMI) and humoral immune response. VLPs bind and activate antigen presenting cells such as DCs, captured by them results in wide range induction of humoral and CMI immune systems including antibodies, T cells (T-helper CD4+ and CD8+ CTL). The particulate nature of VLPs allows them to be taken up by APCs to confer a long-lasting Cytotoxic T-lymphocytes response [18].
Virosomes: Virosomes are virus like particles composed of membrane lipid, viral envelope and glycoproteins, but without genetic material of virus which makes them nonvirulent or cannot cause infection. They can be constructed from diverse enveloped viruses in which they are identical in structures and cell entry characteristics to original virus. Virosomes works both as delivery system for antigens and as adjuvant to stimulate immune response, also called immunopotentioters. Virosomes consists particles of virus with incorporated vaccine antigens and used as delivery system to enhance uptake of antigens by APCs. Some studies reveal that in human medicine virosomes have been used to induce immunity against hepatitis A vaccine and virosomal influenza vaccine also induces the same titer of Hemagglutination Inhibition (HI) induced by conventional type of vaccines [41].
Virosomal adjuvants are likely to present antigen to both MHC class II and MHC class I, capable to trigger humoral (B cells) as well as cell mediated immune response (T cells). Cytokines such as TNF-α, IFN-γ and IL-2 can be induced by virosomes. In general, the importance of using virosomes in vaccines are because of safe (nontoxic), biodegradable, do not react against themselves by producing antibodies, stabilization and prevention of antigen from degradation by PH or enzymatic activities [41,28].
Cytokines: Cytokines are proteins involved in the immune systems to defend against foreign pathogens via B and T lymphocytes activation, proliferation and differentiation. They are natural secretory peptides of immune system used in response against pathogens. Cytokines play an important role in recruitment of immune cells including activation of Natural killer cells (NK), Cytotoxic T lymphocytes and mononuclear phagocytic cells [42]. Current studies have revealed that cytokines to be likely as a potent vaccine adjuvant. They have been developed using recombinant technology to increase the efficacy of vaccination and to use as an alternative adjuvants of vaccine. Recombinant cytokines such as interleukin (IL-1), IL-2, IL-3, IL-6, IL-12, TNF, IF-gamma and granulocyte macrophage colony stimulating factor (GM-CSF) have display the ability to increase vaccine efficacy and considered as adjuvants. However, their function is differing in that some enhance specific immune cells activities, while the others activates general immune cells, moreover, they can induce other cytokines. Cytokines-based adjuvants have been used by incorporating into liposomes and cytokines expression vectors to improve their halflife in vaccine [43].
Among cytokines, IL-2 has been studied widely because of their ability to activate proliferation of T cells. Trials carried out to investigate cytokines using IL-2 as adjuvant with killed rabies vaccine and antigen of herpes simplex virus shows strong immune response in mouse models. IL-2 demonstrated in cats for vaccine of feline immunodeficiency virus subunit resulted as an effective adjuvant and combination of IL-2 with IL-18 in Feline leukemia virus DNA vaccine was also effective [44]. Additionally, effects of IL-2 have been investigated in laboratory animals including guinea pigs, mice, pig and cattle. In immunosuppressed mice efficacy of IF-gamma was reported [45]. IL-12 has been show the ability to induce production of antibodies (IgA and IgG) in administration as mucosal adjuvant, it also activates mucosal immunity. Antigen presenting cells can be also activated and recruited by Granulocytemacrophage colony-stimulating factor [46].
Combined vaccine adjuvant: Vaccination remains an effective tool in preventing and controlling infectious disease of human and animals [9]. Even though development of effective vaccines is highly determined by appropriate adjuvant selection and formulation that able to robust immune response against infectious pathogens. Adjuvants are important components of vaccines for both animals and humans. Majority of existing vaccines are formulated with only single adjuvants, which cannot induce the required level of immune response relative to vaccines contains more than one adjuvants. Because of this limitation several studies are discovering multiple adjuvants formulated with different vaccines to improve efficacy, reduce required antigen and to provide long-lasting immunity [47].
Recent advance of vaccine development results in formulation of combined adjuvants to provoke immune system effectively with less adverse effects on vaccine recipient [48]. Combined adjuvants, contains more than one molecule that act in an interactive manner or synergistically and involve in activation of immune system in various mechanisms. Nowadays several combined vaccine adjuvants are developed in different vaccines of both human and animals [49]. For example, reveals that combination of Toll-like Receptor (TLR) agonist with alum-vaccine complexes may increase the efficiency of the vaccine.
Majority of the available combined adjuvants constitute of delivery system and immunostimulatory adjuvant. Delivery systems have known by having function of both antigen delivery and as adjuvant activity, but its adjuvanticity is not strong enough to activate innate immunity. Delivery systems have tendency to induce Th2-type immune responses, which are weak to confer protection against infectious pathogens, sufficient protection can be achieved through induction of Th2-type with Th1-type response. In contrast, immunostimulatory adjuvants activate innate immune system including Th1 response, however Th1 has short-lived in vivo. To overcome the limitations of delivery system and immunostimulatory adjuvant, their combination is required. Combining them provides significant level of immune response than a single adjuvant. Combination of Various pathogen recognition receptors and delivery systems has been evaluated: TLR-9 and TLR-4 are prominently used [47].
Immuno Stimulating Complexes (ISCOMs), liposomes, Adjuvant Systems Montanides, and triple adjuvant combinations are major combined adjuvants licensed and in development. Liposomes have been used both to delivery antigen to immune cells such as APCs by preventing them from degradation and as adjuvant to enhance immune response. Although liposomes can combined with another compounds or TLR ligands to increase adjuvanticty. ISCOMs containing cholesterol, phospholipid and saponin (Quil-A) have been used in species of veterinary importance including small and large animals. Furthermore, ISCOMs was combined with different vaccines to yield effective immune response, for example with H5N1 influenza vaccine, Cholera Toxin A (CTA) subunit [49].
Montanide is a type of oil based adjuvants that have been used in different species of experimental animals with natural and synthetic or recombinant antigens. Both in veterinary and human vaccines, montanide-based adjuvants have been used. The effect of this oilbased adjuvant in vaccine of Foot-and-Mouth Disease (FMD) shows successful protection in animals and it currently exists on market as adjuvant of Foot-and-Mouth Disease (FMD) vaccine. In case of humans it is in development for HIV, malaria and breast cancer Mohan et al,. Montanide can improve by allowing combination with other types of adjuvants and immunostimulators. Combination of Montanide and ISCOMs were investigated for recombinant vaccine of FMD virus, revealed significant long lasting immunity in Guinea pigs. It has been studied in combination with TLR ligands such as LPS for TLR4, CpG ODN for TLR9, TLR7 and TLR3 [49].
Conclusion
In general, the ultimate goal of vaccination is activation of a powerful and long-term immune response against infectious diseases of animals and human. These effective immunities can be accomplished by using vaccines formulated with proper antigens and adjuvants. Adjuvants are substances added to vaccines to get optimal immune response. Several natural and synthetic adjuvants can be used to increase the efficiency of vaccines. Of these, aluminum compounds, emulsions, saponins, ISCOMs, different products of bacteria, liposomes, cytokine and combined adjuvants have been evaluated. Conventional vaccine formulation was mainly based on single adjuvant, but recently progress of combined adjuvants was revealed. Therefore, understanding the mechanisms of action of these adjuvants plays a crucial role in vaccine development. Nowadays, considerable developments have been done to rise knowledge of mechanisms of various adjuvants in activation of innate immunity. An adjuvant can act as a delivery system, comprise activation of APCs particularly DCs and stimulation of innate immune response. In the development of vaccine adjuvants, it is important to consider safety.
Based on the above conclusion the following recommendations are forwarded: In vaccine development different selection criteria of adjuvants should be considered in order to increase and provide effective vaccine in the target animal species, induce rapid and long-lasting protective immunity and reduce adverse effects of the adjuvants. Further activity and effectiveness of different adjuvants should also be evaluated to achieve the mentioned points including by combination methods, since development of novel adjuvants and adjuvant combinations is likely to help to address the challenges of vaccinology.
REFERENCES
- Pasquale AD, Preiss S, Silva FT, Garçon N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines. 2015;3(2):320-343.
- O'HAGAN DT. Conference Science Medal Lecture: Recent Advances in Vaccine Adjuvants for Systemic and Mucosal Administration. J Pharm Pharmacol. 1998;50(1):1-0.
- Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492-503.
- Lee S, Nguyen MT. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015;15(2):51-57.
- Vogel FR. Improving vaccine performance with adjuvants. Arch Clin Infect Dis 2000;30(Supplement_3):S266-70.
- Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. FRONT IMMUNOL. 2013;4:114.
- Singh M, T O'Hagan D. Recent advances in veterinary vaccine adjuvants. J Parasitol Res. 2003;33(5-6):469-478.
- Aguilar JC, Rodriguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25(19):3752-62.
- Knight-Jones TJ, Edmond K, Gubbins S, Paton DJ. Veterinary and human vaccine evaluation methods. Proceedings of the Royal Society. Res J Biol Sci. 2014;281(1784):20132839.
- Zhou B. Classical swine fever in China-an update Minireview. Frontiers in veterinary science. 2019;6:187.
- Ott G,Van Nest G. Development of Vaccine Adjuvants: A Historical Perspective. In Vaccine Adjuvants and Delivery Systems. 2006.
- Garçon N, Leroux-Roels G, Cheng WF. Vaccine adjuvants. Perspectives in Vaccinology. Vaccines. 2011;1(1):89-113.
- O'Hagan DT, MacKichan ML, Singh M. Recent developments in adjuvants for vaccines against infectious diseases. Biomol Eng. 2001;18(3):69-85.
- Apostólico JD, Lunardelli VA, Coirada FC, Boscardin SB, Rosa DS. Adjuvants: classification, modus operandi, and licensing. J Immunol. Res. 2016;2016.
- Mastelic B, Ahmed S, Egan WM, Del Giudice G, Golding H, Gust I, et al. Mode of action of adjuvants: implications for vaccine safety and design. Res J Biol Sci. 2010;38(5):594-601.
- Spickler AR, Roth JA. Adjuvants in veterinary vaccines: modes of action and adverse effects. J Vet Intern Med. 2003;17(3):273-281.
- Agrawal A, Owais M, Singh UP. Novel Vaccine Adjuvants.
- Azmi F, Ahmad Fuaad AA, Skwarczynski M, Toth I. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Hum Vaccines Immunother. 2014;10(3):778-96.
- Mohan T, Verma P, Rao DN. Novel adjuvants & delivery vehicles for vaccines development: a road ahead. Indian J Med Res. 2013;138(5):779.
- He P, Zou Y, Hu Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum Vaccines Immunother. 2015;11(2):477-88.
- HogenEsch H, O’Hagan DT, Fox CB. Optimizing the utilization of aluminum adjuvants in vaccines: you might just get what you want. NPJ Vaccines. 2018;3(1):1-1.
- Sun B, Ji Z, Xia T. Aluminum-Based Nano-adjuvants. In Encyclopedia of Nanotechnology. 2014.
- Kool M, Fierens K, Lambrecht BN. Alum adjuvant: some of the tricks of the oldest adjuvant. J Med Microbiol. 2012;61(7):927-34.
- Ulmer J. Vaccine adjuvants: mode of action. FRONT IMMUNOL. 2013;4:214.
- Haensler J. Oil emulsions as vaccine adjuvants. Future Medicine Ltd. 2011.
- Shah RR, Brito LA, O’Hagan DT, Amiji MM. Emulsions as vaccine adjuvants. In Subunit Vaccine Delivery 2015;59-76
- Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunology and cell biology. 2004;82(5):488-96.
- Gerdts V. Adjuvants for veterinary vaccines-types and modes of action. Berliner und Munchener tierarztliche Wochenschrift. 2015;128(11-12):456-63.
- Fernández-Tejada A, Tan DS, Gin DY. Development of improved vaccine adjuvants based on the saponin natural product QS-21 through chemical synthesis. Acc Chem Res 2016;49(9):1741-56.
- Garçon N, Hem S, Friede M. Evolution of adjuvants across. Vaccines. 2012; 22:58.
- Ahlberg V, Hjertner B, Wallgren P, Hellman S, Bengtsson KL, Fossum C. Innate immune responses induced by the saponin adjuvant Matrix-M in specific pathogen free pigs. J Vet Res. 2017;48(1):30.
- Sun HX, Xie Y, Ye YP. Advances in saponin-based adjuvants. Vaccine. 2009 ;27(12):1787-1796.
- Rajput ZI, Hu SH, Xiao CW, Arijo AG. Adjuvant effects of saponins on animal immune responses. J Zhejiang Univ Sci B. 2007;8(3):153-61.
- Alving CR, Alan DT, Lawrence RS. Vaccine adjuvants. Vaccines for biodefense and emerging and neglected diseases. Vaccines. 2009;115-29.
- Sjölander A, Cox JC, Barr IG. ISCOMs: an adjuvant with multiple functions. J Leukoc Biol 1998;64(6):713-723.
- Lövgren Bengtsson K, Morein B, Osterhaus AD. ISCOM technology-based Matrix M™ adjuvant: success in future vaccines relies on formulation. Expert Rev Vaccines. 2011;10(4):401-403.
- Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Therapeutic advances in vaccines. 2014;2(6):159-82.
- Wang N, Wu T, Wang T. Liposomes Used as a Vaccine Adjuvant-Delivery System. J Liposome Res. 2017;25:129.
- Tandrup Schmidt S, Foged C, Smith Korsholm K, Rades T, Christensen D. Liposome-based adjuvants for subunit vaccines: formulation strategies for subunit antigens and immunostimulators. Int J Pharm. 2016;8(1):7.
- Kushnir N, Streatfield SJ, Yusibov V. Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine. 2012;31(1):58-83.
- Rambe DS, Del Giudice G, Rossi S, Sanicas M. Safety and mechanism of action of licensed vaccine adjuvants. Int Curr Pharm J. 2015;4(8):420-31.
- Taylor CE. Cytokines as adjuvants for vaccines: antigen-specific responses differ from polyclonal responses. INFECT IMMUN. 1995;63(9):3241-4.
- Tovey M G, Lallemand C. Adjuvant activity of cytokines. Methods in Molecular Biology (Clifton, N.J). 2010.
- Hanlon L, Argyle D, Bain D, Nicolson L, Dunham S, Golder MC, McDonald M, et al. Feline leukemia virus DNA vaccine efficacy is enhanced by coadministration with interleukin-12 (IL-12) and IL-18 expression vectors. J Virol. 2001;75(18):8424-8433.
- Asif M, Jenkins KA, Hilton LS, Kimpton WG, Bean AG, Lowenthal JW. Cytokines as adjuvants for avian vaccines. Immunology and cell biology. 2004;82(6):638-43.
- Lynch JM, Briles DE, Metzger DW. Increased protection against pneumococcal disease by mucosal administration of conjugate vaccine plus interleukin-12. INFECT IMMUN. 2003;71(8):4780-4788.
- Mutwiri G, Gerdts V, van Drunen Littel-van den Hurk S, Auray G, Eng N, Garlapati S, Babiuk LA, Potter A. Combination adjuvants: the next generation of adjuvants?. Expert Rev Vaccines. 2011;10(1):95-107.
- Kaurav M, Madan J, Sudheesh MS, Pandey RS. Combined adjuvant-delivery system for new generation vaccine antigens: alliance has its own advantage. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S818-31.
- Levast B, Awate S, Babiuk L, Mutwiri G, Gerdts V. Vaccine potentiation by combination adjuvants. Vaccines. 2014;2(2):297-322.
Citation: Ayele G (2020) Review on Recent Advance of Vaccine Adjuvants. J Vaccines Vaccin. S5:003.
Copyright: © 2020 Ayele G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


