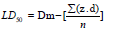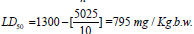PMC/PubMed Indexed Articles
Indexed In
- Open J Gate
- Genamics JournalSeek
- ResearchBible
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Original Research Article - (2020) Volume 9, Issue 2
Resveratrol Nanoemulsion: A Promising Protector Against Ethinylestradiol-Induced Hepatic Cholestasis in Female Rats
Hussein MA*, Kasser AK, Mohamed AT, Eraqy TH and Asaad AReceived: 24-Feb-2019 Published: 27-Mar-2019, DOI: 10.35248/2167-7956.19.8.175
Abstract
Ethinylestradiol (EE) induced cholestasis was reported in female rats-model. The present article aimed to investigate anticholestatic activity of Resveratrol Nanoemulsion (RENE) against EE-induced cholestasis in adult female rats. The mean particle size of RENE was 49.5 ± 0.05 nm and zeta potential of +15.75 with the observed shapes of nanoparticle was spherical. Also, the median lethal dose (LD50) of RENE in rats was 795 mg/kg body weight. Administration of 1/20 LD50 RENE (39.75 mg/kg.b.w.) to EE–treated rats normalize serum cholesterol level as well as against an increase of serum TBA, bilirubin concentration. The treatment also resulted in a significant increase in hepatic SOD and GPx. RENE inhibited serum ALP, ALT and γ-GT activities, as well as reduced serum TNF-α, NO, MMP-2 MMP-9 and hepatic MDA as compared to EE-treated rats. The results clearly suggest that RENE has a powerful prophylactic action in cholestasis induced by EE.
Keywords
Resveratrol nanoemulsion; Ethinylestradiol; Hepatic cholestasis; Nano particles oxidative stress biomarkers; Matrix metalloproteinases
Abbreviations
RENE: Resveratrol Nanoemulsion; EE: Ethinylestradiol; SOD: Superoxide Dismutase; GPx: Glutathione Peroxidase; ALP: Alkaline Phosphatase; ALT: Alanine Aminotransferase; γ-GT: Gamma-glutamyl Transpeptidase; TBA: Total Bile Acids; TNF-α: Tumor Necrosis Factor-alpha; NO: Nitric Oxide; MMP-2: Matrix Metalloproteinase-2; MDA: Malondialdehyde; MMP-9: Matrix Metalloproteinase-9
Introduction
Cholestasis is characterized by oxidative stress, inflammation and disorders of the liver, bile duct and pancreas [1]. When bile flow is stopped, the pigment bilirubin, a waste product that’s formed when old or damaged red blood cells are broken down, escapes into the bloodstream and accumulates [2]. The presence of a lesion in the cellular parenchyma is common in a large number of chronic liver diseases, such as viral hepatitis, alcoholic hepatitis, and cholestasis [3]. Intrahepatic cholestasis evaluated in susceptible women during pregnancy after administration of estrogens [4]. EE is widely used to cause experimental cholestasis in rat’s model [5]. EE down-regulated of Na+-taurocholate cotransporting polypeptide (Ntcp) expression leading to decrease sinusoidal uptake of bile acids [6,7]. In addition, γ-GT levels were raised after the administration of EE in the prophylactic treatment. As, earlier mentioned, γ-GT and ALP are employed to detect impaired bile flow (cholestasis) [8-11]. Among the many drugs for liver injury, resveratrol is the most clinically popular for patients and is known to have hepatotherapeutic and anti-fibrotic properties [5]. Resveratrol has also been proven effective in several research fields, such as protecting against genomic injury, increasing hepatocyte protein synthesis, decreasing the activity of tumor promoters and stabilizing mast cells. Pharmaceutical and medicinal activity of red wine depends on its several factors including the grape variety, vineyard location, cultivation system, climate and soil type and production process among others [12,13]. In addition to its free radical scavenging properties, silymarin increases antioxidant enzymes, such as SOD and catalase and inhibits lipid peroxidation [14,15]. According to many authors however, it has a low bioavailability [16]. Nanotechnology is at the forefront of cancer research. This, technology allows scientists to target cancer cells [17]. Nanoparticles can decrease side effects in patients by directly targeting the area of disease and eliminating the need to circulate through the body [18,19]. When encapsulating drugs into nanoparticles, researchers observed improved drug solubility, controlled drug release, enhanced bioavailability, increased stability and improved long-term storage (versus non-encapsulated drugs) [20]. These attributes are promising and could be the traits needed to combat disease. Not surprisingly, a phenolic compounds such as resveratrol, an excellent scavenger of reactive oxygen species and anticholestatic activity [21-22]. The aim of this study was to investigate in vivo, the anticholestatic and hepatoprotective activities of resveratrol nanoemulsion against EE-induced cholestasis in female rats.
Materials and Methods
Materials
RPMI1640 (Roswell park memorial institute) medium with L-glutamine (Cambrex, Belgium), Resveratrol, Trypan Blue, Bovine serum albumin, Glutaraldehyde 50% (Sigma, USA). RES, EE and Tween 80 were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals used in this study were of the analytical grade.
Resveratrol nano-emulsion preparation
100 mg Bovine Serum Albumin(BSA) is dissolved in 12 ml distilled water; dissolve the resveratrol 24 mg in 24 ml ethanol then add drop wise the ethanolic solution on the BSA solution under stirring (500 rpm); then add 3 ml 11% glutaraldehyde and leave on stirring overnight [23].
Resveratrol nano-emulsion characterization
The crystalline nature and grain size of Resveratrol Nanoemulsion (RENE) was carried out by X- ray Diffraction (XRD) pattern at 25-280C with a D8 Advance X –ray diffractometer (Bruker – Germany) with a nickel (Ni) filtered using CuKα (λ= 1.54184 A0) radiations as an X-ray source. Infrared Spectrum (IR) of sample is registered using Nicolet 6700 (Thermo scientific–USA). The thermal analysis was measured using Thermo Gravimetric Analysis (TGA) TGA -50 (Shimadzu, Japan) Morphology and size of RENE were examined by Scanning Electron Microscope (SEM, JSM- 690, JEOL, Inc., Tokyo, Japan) and Field Emission Transmission Electron Microscopy (FETEM, JSM- 2100F, JEOL Inc.) at an accelerating voltage of 15Kv and 200 Kv.
Animals used
The animals used in the study, Female albino rats weighing around 150-170 g (96 rats; 60 for LD50 estimation and 36 for anti-cholestasis activity) were obtained from the animal house of National Cancer Institute Animal House, Egypt. They were housed in plastic cages with stainless steel covers at the National Cancer Institute Animal House. The animals were housed in groups of 6 in stainless steel cages (34 × 47 × 18 cm) with soft wood shavings as bedding, fed with normal commercial pellet diet (GAFCO, Tema), given water ad libitum. Approval for this study was obtained from the Ethical Review Committee of the Faculty of Applied Medical Sciences, October 6 University, Giza, Egypt (No. 201810/01).
Determination of LD50 of resveratrol nanoemulsion
Resveratrol nanoemulsion was orally administrated in different doses to find out the range of doses which cause zero and 100% mortality of animals. A range doses was determined for each extract. LD50 was determined by administration of resveratrol nanoemulsion in different doses 350, 500, 700, 900, 1100 and 1300 mg/kg orally, in six groups, 10 animals in each. After administration of the tested resveratrol nanoemulsion, animals were observed individually every hour during the first day and every day for 21days. Behavior and clinical symptoms of animals were noted throughout the duration of the experiment. The LD50 was calculated by Finney, method using following formula [24]:
LD50= Dm – Σ (Z. d)/n
Dm = The largest that kill all animals.
Σ = The sum of (z x d).
Z = Mean of dead animals between 2 successive groups.
d = The constant factor between 2 respectively doses.
n = Number of female rats in each group.
Experimental setup
Female rats weighing 150-170 g were divided and housed in six groups of six rats each for 5 days before the start of experiment. All animals had free access to water and food except for a 12 hours fasting period before oral administration of the extract. The treatment groups are described in Table 1 [5].
| Group | Group name | Treatment description |
|---|---|---|
| I | Normal control A | Days 1–15: 3 mL of distilled water, orally |
| II | Normal control B | Days 1–15: 3 mL of tween 80, 1%, orally |
| III | RENE | Days 1–15: oral suspension of (1/20 LD50) 39.75 mg/kg b.w. RENE in tween 80, 1% [5]. |
| IV | EE | Days 1–10: 3 mL of tween 80, 1%, orally [5]. Days 11–15: subcutaneous injection of 100 µg EE/kg b.w. in tween 80, 1%, one dose daily [5]. |
| V | RENE + EE (prophylactic I) |
Days 1–10: oral suspension of 39.75 mg RENE/kg b.w. in tween 80, 1%. Days 11–15: oral suspension of 39.75 mg RENE/kg b.w. in tween 80, 1%; subcutaneous injection of 100 µg EE/ kg b.w. in tween 80, 1%, in a single daily dose |
| VI | EE + RENE (prophylactic II) |
Days 1–10: oral suspension of 39.75 mg RENE/kg b.w. in tween 80, 1%; subcutaneous injection of 100 µg EE/kg b.w. in tween 80, 1%, in a single daily dose Days 11–15: oral suspension of 39.75 mg RES/kg b.w. in tween 80, 1%. |
Table 1: Description of Treatment Groups.
Biochemical assays
Parameters that were determined include: levels of serum level of cholesterol, bilirubin, ALT, ALP and γ-GT were determined using Reflotron Plus Analyzer and Roche kits, respectively. Serum TNF-α was quantitatively estimated by Enzyme-Linked Immunosorbent Assay (ELISA) according to Corti et al., [25-31]. Serum NO was estimated according to the method of Moshage et al. [32]. Finally, plasma levels of MMP-2 and -9 were determined by ELISA kits [33,34] (Abnova, Taipei City, Taiwan).
Measurement of antioxidant enzymes & lipid peroxidation
SOD and GPx were determined in liver tissues by red formazan dye reduction produced and coupled reaction method with GR respectively [35-36]. Also, thiobarbituric acid reactive substances assay kit to measure the liver lipid peroxidation products, MDA equivalents [37].
Histological assessment
Portions of the tissue from liver were used for histopathological examination. Tissues were fixed in 10% buffered formalin (pH 7.2) and dehydrated through a series of ethanol solutions, embedded in paraffin and routinely processed for histological analysis according to the method of Bancroft and Steven [38]. Sections of 2 μm thickness were cut and stained with haematoxylin-eosin for examination. The stained tissues were observed through an Olympus microscope (BX-51) and photographed by a Chare-Couple Device (CCD) camera.
Statistical analysis
Data were presented as mean ± SEM. The presence of significant differences among means of groups was determined by one-way ANOVA using SPSS/18 Software for Windows (USA). P values of <0.05 and 0.01 were considered to indicate statistical significance.
Results
Results showed that the IR spectrum infrared spectrogram of the resveratrol raw powder shows a phenol hydroxyl group absorption peak at 3252 cm-1 and benzene ring absorption peaks at 2827, 2920 exists. Infrared spectrogram of resveratrol nanoemulsion shows a hydroxyl group absorption peak at 3436 cm-1 exists. Light scattering techniques as well as Transmission Electron Microscopy were used to evaluate size, zeta potential and morphology of the nanoformulations. As shown in Figure 1 resveratrol nanoemulsion had size of around 49.5 ± 0.05 nm with negative zeta potential of +15.75. From the equation the LD50 of resveratrol nanoemulsion was calculated to be 795 mg/kg b.w. (Table 2). A single oral dose of resveratrol nanostructure showed increase in heart rate, rapid respiration within 1 to 2 hours at dose levels used (350, 500, 700, 900, 1100 and 1300 mg/kg). The LD50 (rat, oral) is therefore estimated to be beyond 795 mg/kg body weight. The temperature of the animals’ extremities dropped with the toes and tail being cool. Biochemical profiles of the treated animals are presented in Tables 3 and 4. The oral administration of RENE (39.75 mg/kg b.w.) did not cause any significant changes in ALT, ALP, total bilirubin, TBA, γ-GT, TNF-α, NO and cholesterol levels when compared to distilled water and tween 80 control groups. The levels of serum ALT, ALP, total bilirubin, TBA, γ-GT, TNF-α and NO of EE-treated animals were significantly increased. However, there was a significant decrease (p<0.05) of the serum total cholesterol when compared to distilled water and tween 80 control groups. Pre- and post-treatment of animals with RENE significantly reduced ALT, ALP, total bilirubin, TBA, γ-GT, TNF-α and NO levels as well as significantly increased cholesterol (P<0.01), as compared with the EE group. The cholestatic effect of EE was controlled in the rats treated with RENE as demonstrated by the restoration the hepatocytes biomarkers. Also, MMP-2 and -9 are both implicated in the EE-treated rats as compared with the normal control group (P<0.01). Not surprisingly, there were dramatic elevations of the liver MDA and plasma levels of both MMPs as well as deletion of liver SOD and GPx activities in the EE-treated. RENE pre- and posttreatment at RENE significantly (P<0.01) enhanced liver MDA, SOD and GPx as well as plasma MMP-2 and -9 levels as compared to the EE-treated group (Tables 5 and 6).
| Group Number | Dose (mg/kg) |
No. of animals/group | No. of dead animals | (Z) | (d) | (Z.d) |
|---|---|---|---|---|---|---|
| 1 | 350 | 10 | 0 | 0.5 | 150 | 75 |
| 2 | 500 | 10 | 1 | 2.0 | 200 | 400 |
| 3 | 700 | 10 | 3 | 4.0 | 200 | 800 |
| 4 | 900 | 10 | 5 | 6.5 | 300 | 1950 |
| 5 | 1100 | 10 | 8 | 9.0 | 200 | 1800 |
| 6 | 1300 | 10 | 10 | 0 | 00 | 00 |
|
||||||
Table 2: Determination of LD50 of resveratrol nanoemulsion given orally in adult rats.
| Groups | Treatment Description | Cholesterol (mmol/L) |
Bile acids (µmol/L) |
Total bilirubin (µmol/L) |
ALP (ukat/l) |
ALT (U/L) |
|---|---|---|---|---|---|---|
| I | Normal control A (distilled water-treated) 3ml/kg |
1.7 ± 0.23 | 65.15 ± 3.10 | 2.12 ± 0.36 | 10.6± 1.43 | 34.5 ± 2.14 |
| II | Normal control B (tween 80-treated) 3ml/kg |
1.69 ± 0.18 | 60.43 ± 1.54 | 2.35 ± 0.29 | 9.85 ± 2.10 |
30.77 ± 3.56 |
| III | RENE (39.75 mg/kg.b.w.) | 1.88 ± 0.16 | 64.00 ± 5.21 | 2.20 ± 0.40 | 10.45 ± 1.87 |
32.16 ± 2.11 |
| IV | EE (100 µg/kg b.w.) | 0.7 ± 0.09@ | 135.48±8.70@ | 4.2 ± 0.43@ | 22.50 ±2.64@ |
76.43 ± 4.32@ |
| V | RENE + EE (Prophylactic I) |
1.52 ± 0.25@ | 90.64 ± 4.94@ | 2.10 ± 0.04@ | 12.35 ±2.41@ |
42.38 ± 3.25* |
| VI | EE + RENE (Prophylactic II) |
1.30 ± 0.14@ | 110.30±6.40@ | 2.53 ± 0.36@ | 15.40 ± 1.58@ |
53.22 ± 5.09* |
*Values are given as mean ± SD for groups of six animals each. Values are statistically significant at @P<0.01, Values are statistically significant at *P<0.05. Resveratrol nanoemulsion (RENE) and ethinylestradiol (EE) treated rats were compared with normal control B rats. Experimental groups (5&6) were compared with ethinylestradiol (EE) treated rats.
Table 3: Effect of Resveratrol Nanoemulsion (RENE) on serum cholestatic indices in rats treated with ethinylestradiol (EE).
| Groups | Treatment Description | (γ-GT) (U/L) |
TNF-α (pg/ml) |
NO (µmol/L) |
|---|---|---|---|---|
| I | Normal control A (distilled water-treated) 3 ml/kg |
2.07 ± 0.028 | 27.98 ± 2.10 | 19.80 ± 3.08 |
| II | Normal control B (tween 80-treated) 3 ml/kg |
1.97 ± 0.031 | 29.70 ± 3.46 | 18.75 ± 2.63 |
| III | RENE (39.75 mg/kg.b.w.) | 2.11 ± 0.052 | 26.80 ± 2.65 | 20.46 ± 1.46 |
| IV | EE (100 µg/kg b.w.) | 3.68 ± 0.044@ | 58.74 ± 4.11@ | 46.70 ± 4.19@ |
| V | RENE (39.75 mg/kg.b.w).+ EE (100 µg/kg b.w.)(Prophylactic I) |
2.31 ± 0.028@ | 32.65 ± 5.49@ | 29.11 ± 2.76@ |
| VI | EE (100 µg/kg b.w.) + RENE (39.75 mg/kg.b.w.) (Prophylactic II) | 2.43 ± 0.037@ | 35.48 ± 3.22@ | 32.26 ± 3.55@ |
*Values are given as mean ± SD for groups of six animals each. Values are statistically significant at @P<0.01, Values are statistically significant at *P<0.05. Resveratrol Nanoemulsion (RENE) and Eethinylestradiol (EE) treated rats were compared with normal control B rats. Experimental groups (5&6) were compared with Ethinylestradiol (EE) treated rats.
Table 4: Effect of Resveratrol Nanoemulsion (RENE) on activity of Gamma Glutamyl Transpeptidase (γ-GT) as well as levels of Tumor Necrosis Factor alpha (TNF-α), Serum Nitric Oxide (NO) in serum rats treated with Ethinylestradiol (EE).
| Groups | Treatment Description | SOD |
GPx |
MDA (nmol/ mg protein) |
|---|---|---|---|---|
| I | Normal control A (distilled water-treated) 3 ml/kg |
12.05 ± 1.20 | 6.73 ± 0.84 | 1.96 ± 0.04 |
| II | Normal control B (tween 80-treated) 3 ml/kg |
11.97 ± 1.40 | 6.42 ± 0.79 | 1.86 ± 0.08 |
| III | RENE (39.75 mg/kg.b.w.) | 12.80 ± 1.76 | 6.90 ± 0.44 | 1.54 ± 0.05 |
| IV | EE (100 µg/kg b.w.) | 5.48 ± 0.65@ | 3.25 ± 0.38@ | 3.22 ± 0.57@ |
| V | RENE (39.75 mg/kg.b.w).+ EE (100 µg/kg b.w.)(Prophylactic I) |
11.75 ± 1.95@ | 6.21 ± 0.66@ | 2.03 ± 0.39@ |
| VI | EE (100 µg/kg b.w.) + RES (25 mg/kg.b.w.) (Prophylactic II) | 10.70 ± 1.87@ | 5.30 ± 0.42@ | 2.10 ± 0.22* |
* Values are given as mean ± SD for groups of six animals each. Values are statistically significant at @P<0.01, Values are statistically significant at *P<0.05. Resveratrol nanoemulsion (RENE) and ethinylestradiol (EE) treated rats were compared with normal control B rats. Experimental groups (5&6) were compared with ethinylestradiol (EE) treated rats. SOD: one unit of activity was taken as the enzyme reaction, which gave 50% inhibition of NBT reduction in 1min/mg protein; GPx: μg of GSH consumed/min mg protein.
Table 5: Effect of Resveratrol Nanoemulsion (RENE) on activity of Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPx) as well as Malondialdehyde (MDA) level in liver rats treated with Ethinylestradiol (EE).
| Groups | Treatment Description | MMP-2 (ng/ml) |
MMP-9 (ng/ml) |
|---|---|---|---|
| I | Normal control A (distilled water-treated) 3 ml/kg |
28.65 ± 3.25 | 5.80 ± 0.42 |
| II | Normal control B (tween 80-treated) 3 ml/kg |
27.80 ± 2.54 | 5.79 ± 0.31 |
| III | RENE (39.75 mg/kg.b.w.) | 27.50 ± 3.88 | 5.50 ± 0.28 |
| IV | EE (100 µg/kg b.w.) | 65.08 ± 6.50@ | 14.38 ± 2.17@ |
| V | RENE (39.75 mg/kg.b.w).+ EE (100 µg/kg b.w.)(Prophylactic I) |
33.40 ± 2.16@ | 7.59 ± 1.08@ |
| VI | EE (100 µg/kg b.w.) + RES (25mg/kg.b.w.) (Prophylactic II) | 38.17 ± 3.25@ | 10.43 ± 1.70@ |
*Values are given as mean ± SD for groups of six animals each. Values are statistically significant at @P<0.01, Values are statistically significant at *P<0.05. Resveratrol Nanoemulsion (RENE) and Ethinylestradiol (EE) treated rats were compared with normal control B rats.
Table 6: Effect of Resveratrol Nanoemulsion (RENE) on Serum Matrix Metalloproteinase-2 (MMP-2) and Matrix Metalloproteinase-9 (MMP-9) levels in rats treated with Ethinylestradiol (EE).

Figure 1: Resveratrol nanoemulsion had size of around 49.5 ± 0.05 nm with negative zeta potential of +15.75.
Histopathology examination
Gallbladders sections from the distilled water, tween 80 and RENE treated groups had normal appearance and histology (Figure 2a-c). Generally, there were no observable changes in architecture of livers and kidneys of treated animals compared to the control. The histology was consistent with the normal levels of ALT, AST, bilirubin, oxidative stress biomarkers and inflammatory mediators in the serum, plasma and hepatocytes. However, the bile canaliculi, hepatic cell necrosis and higher inflammatory cells infiltrate (stars) were observed in group of female rats treated with EE (Figure 2d). Also, it could be seen that Pre- and post-treatment of animals with RENE significantly reduced bile canaliculi and hepatic cell necrosis compared to the EE-treated group (Figure 2e and f). Results of the gross and histopathological examination are shown in Figure 2.
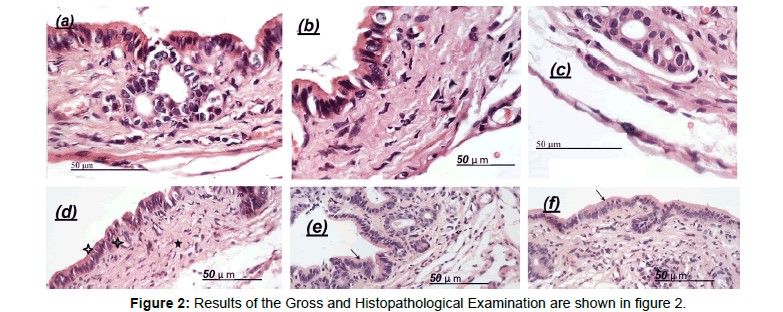
Figure 2: Results of the Gross and Histopathological Examination are shown in figure 2.
Discussion
The liver is a multipurpose organ in the body involved in the regulation of internal chemical environment. Therefore damage to the liver inflicted by a hepatotoxic agent is of critical consequence [5]. The changes is associated with EE-induced liver toxicity are comparable to that of hepatic cholestasis [39,40]. This is due to the infiltration of fatty acids and glycerols into the hepatocytes upon damage to cell membranes [41-43]. Also, the increase in bile flow due to cholesterol precipitation may be responsible for the reduced level of bile acids in serum after EE administration [43]. In agreement with results obtained in similar investigations by Hussein the treatment with EE in the present study elicited a significant decrease in the levels of serum cholesterol as well as increase in the levels of serum, bile acids, ALT, ALP, γ- GT, and BA, total bilirubin, and MDA levels [5]. This was evident in both prophylactic and curative studies. Treatment with the RENE (39.75 mg/kg.b.w.) however significantly increased in the levels of serum cholesterol as well as decrease in the levels of serum, bile acids, ALT, ALP, γ- GT, and BA, total bilirubin, and MDA levels which were elevated by EE- treatment. Total serum bilirubin can also be employed to detect cholestasis in addition to the GGT and ALP and also as a measure of hepatocellular damage [44,45]. In the curative study, total bilirubin levels in the serum of the rats increased significantly in the EE- treated group. This increase was reduced almost to normalcy when treated with the (39.75 mg/kg.b.w.) of RENE. The results of the study suggest that RENE is both proactive and responsive in protecting against EE-induced hepatotoxicity. It is however more bioactive when given to treat an existing damage to the liver. Five days dosing with EE, TNF-α and NO accumulate in serum, detectable as inflammatory mediators, and there is continued produce of free radicals in the endoplasmic reticulum [46,47]. Deregulation of the system can perpetuate inflammatory processes that lead to tissue damage and organ dysfunction [48]. As expected, liver sections from untreated EE –injured rats had higher degree of inflammation and steatosis than the treated groups. Rats that received 100 ug/kg, EE, showed significant cellular degeneration, necrosis and inflammation. Although both TNF-α and NO are commonly thought of as inflammatory mediators because of their high concentrations in liver inflammation and specific for liver lipid peroxidation [49].
The effect with the RENE Pre- and post-treatment was similar in the RENE-treated groups. Higher phase II enzyme activity is expected to be released in rats fed with RENE; mainly SOD and GPx. The possibility of TNF-α and NO to generate ROS during metabolism has been postulated [50]. The ability of RENE to attenuate the hepatotoxic effect of EE could be due to the antioxidant property of RENE may have since EE is known to exert its toxicity through the induction of free radicals [51]. Polyphenols for example are known to be soluble chain breaking inhibitors of the peroxidation process, acting as scavengers of intermediate peroxyl and alkoxyl radicals and chelating metal ions [52]. Prevention of DNA oxidation is also achieved by these polyphenols mainly by quenching free radicals and modulating biometabolism enzymes [5]. Our results indicated that MMP-2 and -9, both involved in rats exposed to EE (100 μg/kg b.w.), were greatly up-regulated in the serum as well as liver of rats with cholestasis. It has been previously shown that reduced maternal MMP-2 levels in the serum is linked to fetal inflammation and preterm labor [5], while increased MMP-2 serum levels are commonly observed in peripartum cardiomyopathy [53]. Furthermore, changes in synthesis of MMP-2 and -9 were associated with pre-eclampsia [54,55]. It was also reported that during defective placental development, the secretions of MMP- 2 and -9 were markedly suppressed, causing intrauterine growth restriction 38. Moreover, the abnormal increase in levels of MMP-2 and -9 on the amnion of fetal membrane was shown to contribute to premature membrane rupture 39 during pregnancy, levels of MMP- 2 and -9 in the serum are tightly regulated, perturbations of which often leads to various maternal and fetal disorders. In the present study, MMP-2 and -9 were found to be up-regulated, suggesting that both MMPs are indeed involved in EE-treated rats, which necessitates further investigations into their functions in the disease. Besides intrahepatic cholestasis of pregnancy, cholestasis is reportedly the major syndrome of hepatotoxicity, in particular those induced by ingestion of harmful herbal products [56,57]. MMPs are also thought to contribute to hepatotoxicity, supported by reports demonstrating the protective effects against hepatotoxicity exerted through downegulation of MMP-9 [58]. On a related note, RENE (39.75 mg/kg b.w.) has been shown to inhibit MMP-9 activity by several independent reports. For instance, resveratrol was shown to inhibit MMP-9 expression in both breast cancer cells and rheumatoid fibroblast-like synoviocytes to suppress their migration and invasion [59]. Given these accumulating evidences supporting the effect of RENE to inhibit MMPs, we aimed to examine whether RENE functioned in a similar fashion in our rat EE-induced ICP model, to suppress both MMPs and thereby ameliorate ICP symptoms. Indeed, treatment with RNEN significantly down-regulated MMP-2 and -9 in the liver as well as in the serum of the ICP rats. It is worth noting that MMP-2 and -9 were may be reduced at both mRNA and protein levels by RNEN treatment, suggesting that the suppression of MMPs by RENE could occur as early as the transcriptional stage. Future investigations are needed to reveal the mechanisms underlying the inhibitory effects of RENE on MMP-2 and MMP-9, as well as to identify potential targets of RENE other than these two MMPs. The histology of the livers showed no signs of toxicity in the I, II control groups of rats. Also, it could be seen that RENE did not show any observable toxicity to the gallbladder compared to the control. From the histopathological observations, it could be seen that EE at the dose of (100 ug/kg) caused severe fatty changes in the livers of rats. In the present study, the histological findings prove that RES affected the recovery of the liver structure in rats with EE-induced liver cirrhosis. Indeed, there was remarkable reduction in fibrosis extent and a decrease of stellate infiltration in rats treated with RENE (39.75kg.b.w.) groups compared to the control EE group. In addition, the most novel and relevant finding was that RENE supplementation was accompanied by the alleviation of bile duct proliferation and ductular reaction in this model. RENE was also able to reduce newly formed bile ducts (Figure 2). Since the proliferation of bile ducts is an early event in cholestasis-related changes, the attenuation of hepatic injury and fibrosis in rats by RES might be associated with alleviation of ductular reaction. Prophylactic effect of RENE against EE-induced cholestasis has not been reported earlier to my knowledge, and this study is perhaps the first observation of its kind.
Conclusion
In summary, we established a rat model of intrahepatic cholestasis induced by ethinylestradiol, with symptoms highly resembling those observed clinically in women with ethinylestradiol. Next, using this rat model, the present study showed that levels of cholestatic indices, oxidative stress biomarkers, MMP-2 and -9 were markedly upregulated in intrahepatic cholestatic rats. Furthermore, the present results showed that the symptoms in the cholestatic rats with EE were significantly ameliorated by RENE. Altogether, our findings in the present study showed that RENE has powerful anticholestatic activity against EE-induced cholestasis, in addition to its antioxidant action and free radical-scavenging activities. Nevertheless, to translate the effects of RENE from animal models to clinical settings, further investigations are required to confirm the efficacy as well as safety of RENE.
REFERENCES
- Sreejayan N, Von Ritter C. Lipid peroxidation in bile: the role of hydrophobic bile acids and the effect on biliary epithelial cell function. Patophysiology. 1999;5:225-232.
- https://www.wiley.com/en-us/Diseases+of+the+Liver+and+Biliary+Sy stem%2C+11th+Edition-p-9780470986813
- Rutherford Ae, Pratt DS. Cholestasis and cholestatic syndromes. Curr Opin Gastroenterol. 2006;22:209-214.
- Zhao Y, Zhai D, He H, Liu J, Li T. Matrine improves 17a-ethinylestradiol- induced acute cholestasis in rats. Hepatol Res. 2009;39:1144-1149.
- Hussien MA. Prophylactic effect of resveratrol against Ethinylestradiol- Induced Liver Cholestasis. J Med Food. 2013;16:246-254.
- Eloranta JJ, Jung D, Kullak-Ublick GA. The Human Na+-taurocholate cotransporting polypeptide gene is activated by glucocorticoid receptor and peroxisome proliferator-activated receptor-gamma coactivator- 1alpha, and suppressed by bile acids via a small heterodimer partner- dependent mechanism. Mol Endocrinol. 2006;20:65-79.
- Lai K, Harnish DC, Evans MJ. Estrogen receptor alpha regulates expression of the orphan receptor small heterodimer partner. J Biol Chem. 2003;278:36418-36429.
- Leung KC, Johannsson G, Leong GM, Ho KK. Estrogen regulation of growth hormone action. Endocr Rev. 2004;25:693-721.
- Waxman DJ, O'Connor C. Growth hormone regulation of sex- dependent liver gene expression. Mol Endocrinol. 2006;20:2613-2629.
- Ameen C, Linden D, Larsson BM, Mode A, Holmang A. Effects of gender and GH secretory pattern on sterol regulatory element-binding protein1c and its target genes in rat liver. Am J Physiol Endocrinol Metab. 2004;287:E1039-1048.
- Simon FR, Fortune J, Iwahashi M, Qadri I, Sutherland E. Multihormonal regulation of hepatic sinusoidal Ntcp gene expression. Am J Physiol Gastrointest Liver Physiol. 2004;287:G782-794.
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nat Rev Drug Discov. 2006;5:493-506.
- Hussein MA, Soad M. Protective effect of Jasonia Montana against ethinylestradiol-induced cholestasis in rats. SPJ. 2008;18:27-33.
- Alptekin N, Mehmetcik G, Uysal M, Aykac-toker G. Evidence for oxidative stress in the hepatic mitochondria of bile duct ligated rats. Pharmacol Res. 1997;36:243-247.
- Guerrero RF, García-Parrilla MC, Puertas B, Cantos-Villar E. Wine, resveratrol and health: A review. Nat Prod Commun. 2009;4:635-658.
- Zhi Z, Matthias F, Mark L, Robert S, Liu Y. Polyphenols from Camellia sinenesis attenuate experimental cholestasis-induced liver fibrosis in rats. Am J Physiol Gastrointest. 2003;285:G1004-G1013.
- Kreuter J. Nanoparticles-a historical perspective. Int J Pharm. 2007;331:1-10.
- De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3:133-149.
- Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310-1316.
- Ibrahim WM, Al-Omrani AH, Yassin AE. Novel sulpiride-loaded solid lipid nanoparticles with enhanced intestinal permeability. Int J Nanomedicine. 2013;9:129-144.
- Hussein MA. A convenient mechanism for the free radical scavenging activity of resveratrol. Int J Phytomed. 2011;3:459-469.
- Majid M, Fatemeh H, Mohamad R. Comparison of effect of resveratrol and vanadium on diabetes related dyslipidemia and hyperglycemia in streptozotocin induced diabetic rats. Adv Pharmaceut Bull. 2011;2:81- 86.
- Albrecht MA, Evans CW, Raston CL. Green chemistry and the health implications of nanoparticles. J Green Chem. 2006;8:417-432.
- https://onlinelibrary.wiley.com/doi/abs/10.1002/bimj.4710210714
- Rishmond W. Total blood cholesterol: A sample method of obtaining blood samples and determining the total cholesterol. Clin Chem. 1973;19:1350-1356.
- Mashige F, Tanaka N, Maki A, Kamei S, Yamanaka M. Direct spectrophotometry of total bile acids in serum. Clin Chem. 1981;27:1352-1356.
- https://scialert.net/fulltextmobile/?doi=ajb.2007.136.141
- Reitman S, Frankel A. A colorimetric method for the determination of serum glutamic oxaloacetic acid and glutamic pyruvic transaminases. Am J Clin Pathol. 1975;28:56-62.
- King EJ, Armstrong. AR: Calcium, phosphorus and phosphate. In: Practical Clinical Biochem. 1988;6: 458.
- Szasz G. A kinetic photometric method for serum gamma glutamyl transferase. Clin Chem. 1969:15:124-136.
- Corti A, Fassino J, Marcucci F, Barbenti E, Cassani G. Oligometric tumor necrosis factor-a slowly converts into the reactive forms at bioactive levels. Biochem J. 1992;284:905-910.
- Moshage H, Rok B, Huizenga R, Jansen P. Nitrite and nitrate determination in plasma: a critical evaluation. Clin Chem. 1992;41:892- 896.
- Lee JG, Dahi S, Mahimkar R, Tulloch NL, Alfonso-Jaume MA. Intronic regulation of matrix metalloproteinase-2 revealed by in vivo transcriptional analysis in ischemia. Proc Nat Acad Sci. 2005;102: 16345-16350.
- Turner HE, Nagy Z, Esiri MM, Harris A, Wass JAH. Role of matrix metalloproteinase 9 in pituitary tumor behavior. J Clin Endocr Metab. 2000;85:2931-2935.
- Suttle N. Copper deficiency in ruminants; recent developments. Vet Rec. 1986;119: 519-22.
- Paglia D, Valentine W. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158-169.
- Esterbauer H, Schaure R, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81-128.
- https://www.scirp.org/(S(lz5mqp453edsnp55rrgjct55))/reference/ReferencesPapers.aspx?ReferenceID=2177753
- Higuchi H, Bronk SF, Taniai M, Canbay A, Gores GJ. Cholestasis increases tumor necrosis factor-related apoptotis-inducing ligand (TRAIL)-R2/DR5 expression and sensitizes the liver to TRAIL- mediated cytotoxicity. J Pharmacol Exp Ther. 2002;303:461-467.
- Nikitin I, Dushkin M, Dologov A, Gordienko I. Effect of 17 a-ethinylestradiol on activity of enzymes synthesis hydrolyzing cholesterol esters in rat liver. Bull Exp Biol Med. 1989;102:162-164.
- Bertolotti R, Spady D. Effect of hypocholesterolemic doses of 17a-ethinylestradiol on cholesterol balance in liver and extrahepatic tissues. J Lipid Res. 1996;37:1812-1822.
- Chao Y, Windler E, Chen C, Havel R. Hepatic catabolism of rat and human lipoprotein in rats treated with 17 a-ethinylestradiol. J Biol Chem. 1979;254:11360-11366.
- Portincasa P, van Erpecum K, Vanberge-Henegouwen G. Cholesterol crystallization in bile. Gut. 1997;41:138-141.
- Brian P, Laden, Todd D, Porter. Resveratrol inhibits human squalene monooxygenase. Nutr Res. 2001;21: 747-753.
- Shen A, Porter T, Wilson T, Kasper B. Structural analysis of the FMN binding domain of NADPH cytochrome P-450 oxidoreductase by site- directed mutagenesis. J Biol Chem. 1989;264:7584-7589.
- Kawamura K, Kobayashi Y, Kageyama F, Kawasaki T, Nagasawa M. Enhanced hepatic lipid peroxidation in patients with primary biliary cirrhosis. Am J Gastroenterol. 2000;95:3596-3601.
- Baroni GS, D’Ambrosio L, Ferretti G, Casini A, Sario AD. Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology. 1998;27:720-726.
- Aggarwal B, Shishodia S, Sandur S, Pandey M, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2007;72:1605- 1621.
- Kundu J, Surh Y. Molecular basis of chemoprevention by resveratrol: NF-kappaB and AP-1 as potential targets. Mutat Res. 2004;555:65-80.
- Strater J, Moller P. Pathogenesis of primary biliary cirrhosis: CD 95-induced apoptosis at last. Eur J Gastroenterol Hepatol. 1998;10:553- 557.
- Fox E, Kim J, Tracy T. NF-Kappa b activation and modulation in hepatic macrophages during cholestatic injury. J Surg Res. 1997;72:129-134.
- Leonard S, Xia C, Jiang B, Stinefelt B. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commu. 2003:309:1017-1026.
- Forster O, Hilfiker-Kleiner D, Ansari AA, Sundstrom JB, Libhaber E. Reversal of IFN-gamma, oxLDL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. Eur J Heart Fail. 2008;10:861-868.
- Montagnana M, Lippi G, Albiero A, Scevarolli S, Salvagno GL. Evaluation of metalloproteinases 2 and 9 and their inhibitors in physiologic and pre-eclamptic pregnancy. J Clin Lab Anal. 2009;23:88- 92.
- Wang Z, Lu S, Liu C, Zhao B, Pei K. Expressional and epigenetic alterations of placental matrix metalloproteinase 9 in preeclampsia. Gynecol Endocrinol. 2010;26:96-102.
- Tarantino G, Pezzullo MG, di Minno MN, Milone F, Pezzullo LS. Drug- induced liver injury due to natural products used for weight loss: a case report. World J Gastroenterol. 2009;15:2414-2417.
- Viswanathan L, Patel A. Hepatotoxicity Associated with Herbal Tea Containing Kelp. ACG Case Rep J. 2013;1:55-57.
- Al-Olayan EM, El-Khadragy MF, Aref AM, Othman MS, Kassab RB. The potential protective effect of Physalis peruviana L. against carbon tetrachloride-induced hepatotoxicity in rats is mediated by suppression of oxidative stress and downregulation of MMP-9 expression. Oxid Med Cell Longev. 2014; 23:381-413.
- Chen Z, Hu L, Lu M, Shen Z. Resveratrol reduces matrix metalloproteinases and alleviates intrahepatic cholestasis of pregnancy in rats. Can J Physiol Pharmacol. 2016;94:402-407.
Citation: Hussein MA, Kasser AK, Mohamed AT, Eraqy TH, Asaad A (2019) Resveratrol Nanoemulsion: A Promising Protector Against Ethinylestradiol-Induced Hepatic Cholestasis in Female Rats. J Biomol Res Ther, 8: 175. doi: 10.35248/2167-7956.19.8.175
Copyright: Ã?â??Ã?© 2019 Hussein MA, et al. This is an open access article distributed under the term of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.