Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- The Global Impact Factor (GIF)
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 10, Issue 2
Quantitative Determination of Brexpiprazole by RP-HPLC Method
Veera S. Pulusu1*, Krishna C. Routhu2 and Soma SB. Chikkaswamy32Pharmaceutics, GITAM Institute of Pharmacy, GITAM University, Visakhapatnam, Andhra Pradesh, India
3Department of Chemistry, University of Mysore, Manasagangotri, Mysore, India
Received: 19-Apr-2019 Published: 29-May-2019, DOI: 10.35248/2153-2435.19.10.610
Abstract
A stability-indicating RP-HPLC method was established and validated for the determination of Brexpiprazole in bulk drug using C18 column Waters (150 mm×4.6 mm, 5 µm) with a mobile phase containing of 500 mL of 10 mM monobasic Potassium phosphate buffer adjusted pH 2.0 with 85% Orthophosphoric acid and 500 mL of HPLC grade Acetonitrile. The mobile phase was filtered with a 0.45 µm membrane filter and degassed by sonicating for few minutes. The detection was performed at 213 nm using a Photodiode Array Detector at a flow rate of 1.0 mL min-1 and Brexpiprazole was eluted at 2.5 min. with column temperature 30°C. The detector response was generated from the concentration range of 0.01-0.06 mg mL-1 and regression coefficient(r) was 0.999. Brexpiprazole was exposed to stress conditions such as acidic, basic, oxidation, hydrolysis, photolysis and thermal degradation, and the outcomes showed that the molecule was more sensitive to peroxide degradation. This method was validated as per ICH and FDA guidelines and showed linearity, accuracy, precision, specificity, robustness, LOD, LOQ and system suitability results within the acceptance criteria.
Introduction
Brexpiprazole is a serotonergic-noradrenegic-dopaminergic acting compound. It is a small molecule with molecular formula C25H27N3O2S, molecular weight 433.57 g/mol and chemically known as 7-{4-[4-(1-Benzothiophen-4-yl)piperazin-1-yl]butoxy} quinoline-2(iH)-one Brexpiprazole is non-hygroscopic, with white to off white crystalline powder and a melting point of 183°C (decomposition) [1]. This is a weakly basic compound with a pKa of 7.8 and this drug is not soluble in water. Brexpiprazole (Figure 1) is used in treatment of schizophrenia and adjunctive treatment of Major Depressive Disorder (MDD) [2]. It has partial agonistic activity at serotonegic 5-HT1A and at dopaminergic D2 receptors, antagonistic activities at serotonergic 5-HT2A, and antagonist activity at noradrenergic α1/2 receptors [2].
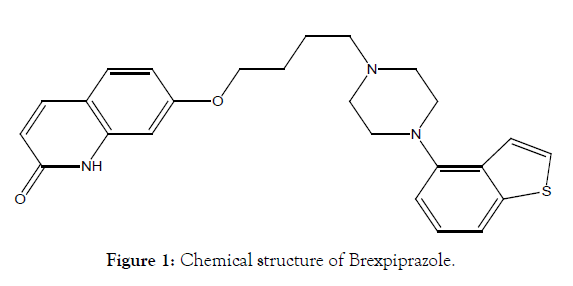
Figure 1: Chemical structure of Brexpiprazole.
Literature survey revealed that Brexpiprazole was determined by UV-visible spectroscopy [3] and HPLC [4-7]. In the current work, the authors have proposed a simple, specific, valid and robust RP-HPLC method for the estimation of Brexpiprazole in pharmaceutical active substance form.
Methodology
Chemicals and solvents
Brexpiprazole standard (Purity ≥ 99.7 as is basis), Acetonitrile (HPLC grade), HPLC grade water (Millipore), Potassium phosphate monobasic, 85% Orthophosphoric acid, Sodium hydroxide, Hydrochloric acid and Hydrogen peroxide were purchased from Merck.
Instrumentation
The instrument employed in the present work were analytical micro balance (Make: Mettler Toledo, Model: XP56), sonicator (Make: Elma, Model: S300H), hot air oven (Make: Servewell Instruments, Model: H02436), digital pH meter (Make: Mettler Toledo) and UVVisible chamber (Make: Mack Equipment, Model: MK-2). HPLC (Waters, 2695 with PDA detector 2996) and empower software.
Method development
Optimization of the chromatographic conditions: Several mobile phases containing Acetonitrile, different pH buffers in different proportions were tried with different column temperature. Mobile phase was prepared by combining 500 mL of 10 mM monobasic Potassium Phosphate buffer, pH was adjusted to 2.0 with 85% Orthophosphoric acid and 500 mL of Acetonitrile. The mobile phase was filtered and de-gassed by sonication. Symmetrical peak was found with Waters C18 column (150 mm×4.6 mm, 5 μm) at 30°C. The flow rate was 1.0 mL/min and wavelength was set at 213 nm. All determinations were performed at ambient temperature for a run time of 5 min. with 10 μL injection volume.
Preparation of working standard solution: The standard solution of Brexpiprazole prepared as 20 mg of pure drug was dissolved in 50 mL volumetric flask and diluted to volume with mobile phase (Stock concentration 0.4 mg mL-1). Further 5.0 mL of stock was diluted to 50 mL with mobile phase and mixed (working std. concentration 0.04 μg mL-1). The representative chromatogram and peak purity curve of Brexpiprazole were shown in Figures 2 and 3 for the proposed method.
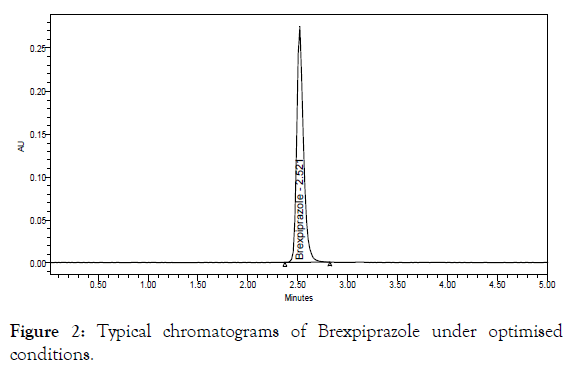
Figure 2: Typical chromatograms of Brexpiprazole under optimised conditions.
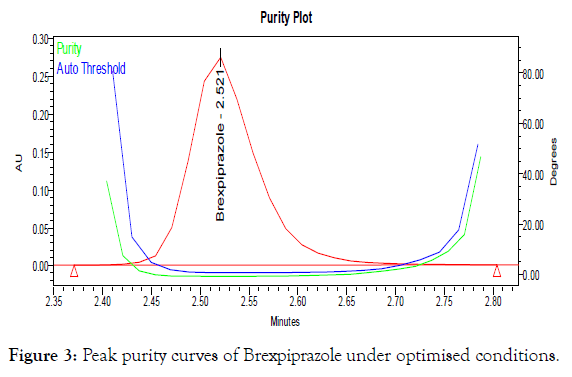
Figure 3: Peak purity curves of Brexpiprazole under optimised conditions.
Method validation
The current developed method was validated according to ICH and FDA guidelines by evaluating linearity, precision (method and intermediate), accuracy, LOD, LOQ, degradation and robustness.
Specificity: Forced degradation study was established by exposing drug substance samples to various stress conditions. Stressed samples were evaluated, active peak was checked for the retention time, peaks interference and spectra purity.
Linearity: A stock solution of Brexpiprazole of 400 μg mL-1 was prepared using the mobile phase as solvent. From the above stock, a series of solutions were prepared from 10 to 60 μg mL-1. In this experiment triplicate injections of each concentration were analyzed and linearity graph was plotted Brexpiprazole concentration vs. peak area.
Accuracy: The accuracy of the developed method was achieved by the addition of known amounts of drug by weight in triplicate (50%, 100% and 150%) around the test concentration, by nine determinations (triplicate preparations of each level). The percentage accuracy and the RSD were calculated for each level to measure how close the experimental value is to the true value.
Precision: Precision was evaluated at two levels, method precision and intermediate precision. Method precision or repeatability was determined by six assay determinations at the 100% test concentration level on the same day. The RSD of obtained results was calculated to estimate method precision results.
Intermediate precision (inter-assay variation) was determined by doing another analyst on a different day and using different equipment. The RSD of combined results obtained by both analysts were calculated to evaluate intermediate precision.
Detection limit and quantification limit:
The Detection Limit (DL) may be expressed as: DL=3.3 σ/S [8]
The Quantification Limit (QL) may be expressed as: QL=10 σ/S [8]
Where, σ is the standard deviation of the response, and “S” is the slope of the calibration curve [8].
The slope S may be estimated from the calibration curve of the analyte. The estimation of σ may be carried out with based on standard deviation of the blank or based on the calibration curve [8].
Robustness: Robustness was established by applying small changes of the following method conditions
1. Flow rate: ±0.2 mL min-1
2. Column temperature: ±5°C
3. Variation in mobile phase organic composition ±10% (absolute)
Standard solution was analyzed for each variation. Change was made to estimate its effect on the developed method. Acquired data for each case was evaluated by calculating % RSD, Retention Time (RT), USP tailing and plate count.
Forced degradation studies
The International Conference on Harmonization (ICH) Q1B guideline [9] provides guidance for performing photostability testing of new drug substances and products. Typical forced stress tests include four main degradation procedures: hydrolytic, oxidative, heat and photolytic degradation [10]. Selecting suitable reagents such as the concentration of acid, base and varying the conditions and length of exposure can achieve the ideal level of degradation [11]. The active substance was exposed to these conditions, and the main peak studied for the peak purity, thus including that the method efficiently separated the degradation products from the active substance. Typical overlay of chromatogram obtained following the assay of stressed samples is shown in (Figure 4).
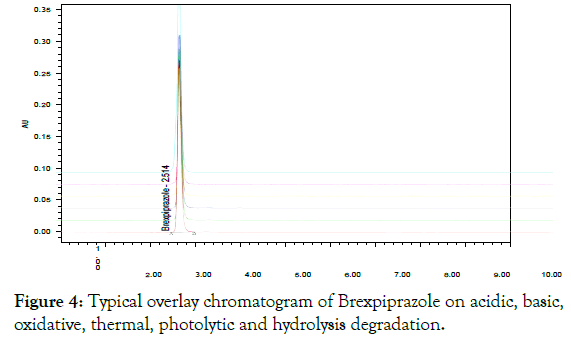
Figure 4: Typical overlay chromatogram of Brexpiprazole on acidic, basic, oxidative, thermal, photolytic and hydrolysis degradation.
Acid and base hydrolysis degradation: Acid and base stress study was performed to force the degradation of the drug substance to its potential degradation products by exposure to acidic, basic and neutral conditions. Hydrolysis is the chemical breakdown of substances by water and depends on the chemistry, solubility, pH, and the oxidation reduction potential of a compound. The possible functional groups undergo hydrolysis are amides, esters, alcohols and imines [10]. Hydrolysis of an amide breaks the carbon-nitrogen bond and produces an acid and either ammonia or an amine. Ester hydrolysis occurs comparatively easily, but amides resist hydrolysis.
Acidic hydrolytic stress of Brexpiprazole was performed using 0.1 M Hydrochloric acid. Weighed and transferred about twenty micrograms of Brexpiprazole active substance into a 50 mL volumetric flask. Added 5.0 mL of 0.1 M HCl and kept in water bath at 80°C for 5 hours. After that immediately neutralized with 5.0 mL of 0.1 M Sodium hydroxide and attained to room temperature. Dissolved and diluted to volume with mobile phase and mixed. Further diluted 5.0 mL to 50 mL with mobile phase and mixed. The obtained chromatogram was analyzed for any degradation occurred during the process. The results are summarized in Table 1.
| Sr. No | Name of Degradation | RT (min) | Area | Purity angle | Purity threshold | % Degradation |
|---|---|---|---|---|---|---|
| 1 | Acid degradation | 2.514 | 1229713 | 0.171 | 0.298 | 4.6 |
| 2 | Base degradation | 2.522 | 1235426 | 0.159 | 0.303 | 3.8 |
| 3 | Peroxide degradation | 2.522 | 1208131 | 0.176 | 0.300 | 6.1 |
| 4 | Thermal degradation | 2.527 | 1259620 | 0.223 | 0.345 | 2.0 |
| 5 | UV degradation | 2.526 | 1276153 | 0.210 | 0.340 | 0.8 |
| 6 | Hydrolysis degradation | 2.518 | 1285933 | 0.259 | 0.302 | 0.2 |
Table 1: Results of degradation.
Base degradation of Brexpiprazole was performed using 0.1 M Sodium hydroxide. Weighed about twenty micrograms of Brexpiprazole active substance and transferred into a 50 mL volumetric flask. Added 5.0 mL of 0.1 M NaCl and kept in water bath at 80°C for 5 hours. After that immediately neutralized with 5.0 mL of 0.1 M HCl and attained to room temperature. Dissolved in and diluted to volume with mobile phase and mixed. Further diluted 5.0 mL to 50 mL with mobile phase and mixed. The obtained chromatogram was analyzed for any degradation occurred during the process. The results are given in Table 1.
Oxidation degradation: Different concentration of Hydrogen peroxide is generally using in oxidative stress study. Although hydrogen peroxide is used mostly because it mimics possible presence of peroxides in excipients, other oxidizing agents such as metal ions, oxygen, and radical initiators can be used [11]. Selection of oxidizing agents, its concentration, and conditions depends on the nature of the drug substance [11].
Peroxide degradation of Brexpiprazole was performed using 3% Hydrogen peroxide. Weighed about twenty micrograms of Brexpiprazole drug substance and transferred into a 50 mL volumetric flask. Added 5.0 mL of 3% Hydrogen peroxide and kept in water bath at 60°C for 3 hours. After the attained room temperature, dissolved in and diluted to volume with mobile phase, and mixed. Further diluted 5.0 mL to 50 mL with mobile phase and mixed. Injected immediately into HPLC to avoid further degradation. The obtained chromatogram was analyzed for any degradation occurred during the process. The results are given in Table 1.
Photolytic degradation: Photostability stress study should be an essential part of degradation, especially for photo-labile molecules. Samples of active drug substance, and solid drug product, should be exposed to a minimum of 1.2 million lux hours and 200-watt hours per square meter light [11]. The same sample should be exposed to both white and UV light. Temperature control may be necessary to minimize the effect of temperature changes during exposure [11].
Brexpiprazole drug substance was exposed to light about 1.2×106 lux-hours. The exposed drug was weighed about twenty micrograms and transferred into a 50 mL volumetric flask. Dissolved in and diluted to volume with mobile phase, and mixed. Further diluted 5.0 mL to 50 mL with mobile phase and mixed. The obtained chromatogram was analyzed for any degradation occurred during the process. The results are given in Table 1.
Thermal degradation: Thermal degradation testing conditions should be higher than recommended ICH Q1 Aaccelerated testing conditions [12]. Samples of solid-state drug substances and finished products should be exposed to dry heat and wet heat, whereas liquid drug products can be exposed to dry heat. Thermal degradation study is recommended that the effect of temperature be studied in 10°C gradual increment above that for routine accelerated testing, and humidity at 75% relative humidity or greater [11].
Thermal degradation was performed by treating the Brexpiprazole drug substance at 40°C/75% RH for 14 days in an open container. Weighed about twenty micrograms of above treated Brexpiprazole drug substance and transferred into a 50 mL volumetric flask. Dissolved in and diluted to volume with mobile phase, and mixed. Further diluted 5.0 mL to 50 mL with mobile phase and mixed. The obtained chromatogram was analyzed for any degradation occurred during the process. The results are given in Table 1.
Hydrolysis degradation: Hydrolysis degradation of Brexpiprazole drug substance was performed using distilled water. Weighed about twenty micrograms of Brexpiprazole substance and transferred into a 50 mL volumetric flask. Added 5.0 mL of distilled water and dissolved the drug. Then the volumetric flask was heated in a water bath at 80°C for 3 hours. After attaining to room temperature, flask was diluted to volume with mobile phase and mixed. Further diluted 5.0 mL to 50 mL with mobile phase and mixed. The obtained chromatogram was analyzed for any degradation that could have occurred during the process. The results are given in Table 1.
Results
Method development
The developed chromatographic conditions were optimized to determine Brexpiprazole in drug substance and dosage form. Symmetrical peak was found with Waters C18 (150 mm×4.6 mm, 5 μm) column at 30°C and mobile phase was consisted of Acetonitrile: 10 mM monobasic potassium phosphate buffer, pH 2.0 with 85% Orthophosphoric acid (50:50, %). Detector performed at 213 nm and flow rate was 1.0 mL/min. The injection volume was 10 μL and the run time was 5 min.
Method validation
Linearity: The linearity was performed using a calibration curve to check the capability of the analytical method to get a proportional response to the analyte concentration in the sample. The working sample (Method & Intermediate precision) concentration and samples tested for accuracy were in the linear range. Standard stock was used as linearity stock and prepared 0.01 to 0.06 mg mL-1 solutions. The linearity was performed at six different concentrations in triplicate. The regression equation was determined as y=3.1x+9163.4 and regression coefficient (r) was 0.999.
Precision: The Method precision and Intermediate precision (ruggedness) was prepared and evaluated six samples at 100% of the target sample concentration as per method. The results of % assay and % RSD are presented in Table 2.
| Sample no. | Conc. (µg/mL) | Method Precision | Intermediate precision | ||
|---|---|---|---|---|---|
| Peak area | % Assay | Peak area | % Assay | ||
| 1 | 40 | 1298524 | 100.0 | 1280779 | 99.9 |
| 2 | 40 | 1281184 | 99.7 | 1283582 | 99.8 |
| 3 | 40 | 1284808 | 99.8 | 1286481 | 100.1 |
| 4 | 40 | 1277418 | 99.8 | 1281706 | 100.0 |
| 5 | 40 | 1276845 | 99.7 | 1282942 | 99.8 |
| 6 | 40 | 1283383 | 99.8 | 1285584 | 99.9 |
| Average | 99.8 | 99.9 | |||
| % RSD | 0.10 | 0.10 | |||
Table 2: Results of method precision and intermediate precision (ruggedness).
Accuracy: The recovery of the method was determined by analyzing samples at three different levels of concentrations by spiking Brexpiprazole active substances. The % recovery results are showed in below Table 3.
| Sr. No | Recovery level % |
Mg Added (spiked) |
mg Found | % Recovery | Mean | SD | % RSD |
|---|---|---|---|---|---|---|---|
| 1 | 50%-1 | 9.99 | 9.964 | 99.7 | 99.8 | 0.1155 | 0.12 |
| 2 | 50%-2 | 9.96 | 9.948 | 99.9 | |||
| 3 | 50%-3 | 10.02 | 9.991 | 99.7 | |||
| 4 | 100%-1 | 19.64 | 19.637 | 100.0 | 99.9 | 0.1528 | 0.15 |
| 5 | 100%-2 | 19.74 | 19.688 | 99.7 | |||
| 6 | 100%-3 | 19.73 | 19.706 | 99.9 | |||
| 7 | 150%-1 | 29.28 | 29.243 | 99.9 | 99.9 | 0.0577 | 0.06 |
| 8 | 150%-2 | 29.42 | 29.385 | 99.9 | |||
| 9 | 150%-3 | 29.47 | 29.461 | 100.0 |
Table 3: Recovery of Brexpiprazole drug substance summary.
This method was demonstrated to be accurate for the range from 50% to 150% of the sample concentration.
LOD and LOQ: The Limit of Detection (LOD) is the lowest concentration of analyte in a sample that can be detected, but not necessarily quantitated [8], while the Limit of Quantification (LOQ) is the lowest concentration of analyte in a sample that can be determined with acceptable precision and accuracy [8]. The current method showed a LOD of 0.10 μg mL-1 and LOQ of 0.30 μg mL-1 respectively and indicating method was extremely rugged.
Forced degradation studies
These stress conditions produce minute or no degradation due to the nature of a drug molecule. The nature of degradation depends on the functional groups of the drug molecule and the effect of stress conditions.
Robustness
Robustness of the method was studied by small variation of the chromatographic conditions such as flow (1.0 ± 0.2 mL/min), column temperature (30 ± 5°C) and mobile phase composition (±10% absolute). The results are given in Table 4.
| Parameters | Modification | RT (min) | Area | USPP late count | USP tailing |
|---|---|---|---|---|---|
| Flow rate | 0.8 mL/min | 2.833 | 1460308 | 6983 | 1.16 |
| Optimized | 2.524 | 1290266 | 6569 | 1.18 | |
| 1.2 mL/min | 2.283 | 1163934 | 6659 | 1.16 | |
| Mobile phase composition | 5% less | 2.498 | 1294719 | 6477 | 1.17 |
| Optimized | 2.524 | 1290266 | 6569 | 1.18 | |
| 5% more | 2.555 | 1303388 | 6651 | 1.18 | |
| Column temperature | 25°C | 2.546 | 1312629 | 6764 | 1.17 |
| Optimized | 2.524 | 1290266 | 6569 | 1.18 | |
| 35°C | 2.505 | 1310603 | 6648 | 1.19 | |
| Note: RT (min), area counts, USP plate count and USP tailing values are in average | |||||
Table 4: Robustness data of the RP-HPLC method at different flow rates for Brexpiprazole.
Variation in flow rate, mobile phase composition and column temperature do not affect the ability of the method to meet system analysis requirements (plate counts and tailing factor) and none of the above variations resulted in critical changes to the system analysis parameters.
Discussion
In this RP-HPLC method, the linearity was within the range of 10- 60 μg mL and regression equation were found to be y=3.1x+9163.4 with regression coefficient (r) 0.999. The method was successfully validated in the optimized conditions, and the validation parameters were within the specified limits. In this developed and validated RP-HPLC method, the LOD and LOQ of Brexpiprazole were found to be 0.1 μg mL-1 and 0.3 μg mL-1, respectively.
The RSD in precision experiment was found to be 0.10% (Intraday) and 0.10% (Inter-day). The % RSD in accuracy and robustness experiments was found to be less than 2.0%, indicating that the method is precise and accurate.
The theoretical plates were above 6500 (more than 2000) and tailing factor was 1.19 (less than 2.0) for Brexpiprazole peak. The % RSD value of assay determination was less than 2.0%, indicating that the developed method was robust.
In the current experiment, degradation of Brexpiprazole was carried out as per the ICH guidelines. The result of peroxide stress showed that 6.1% of degradation of the drug occurred when the drug substance was treated with 3% peroxide and kept in water bath at 60°C for 3 hours. In the hydrolysis degradation study, Brexpiprazole showed no degradation at 80°C for 3 hours.
Conclusions
This study is an example of development of a stability indicating assay, established following the recommendations of ICH/FDA guidelines.
The proposed method showed acceptable precision, accuracy, sensitivity (LOD and LOQ) and wide linear concentration range. The established RP-HPLC method was found to be reliable for the analysis of Brexpiprazole, and was found to be simple, consistent, cost-effective and precise.
Therefore, this RP-HPLC method for determination of Brexpiprazole can be used in quality control or routine for its quantitative determination in bulk and pharmaceutical dosage form.
REFERENCES
- Diefenderfer LA, Iuppa C. Brexpiprazole: A review of a new treatment option for schizophrenia and major depressive disorder. Ment Health Clinician. 2017;7:207-212.
- Citrome L, Stensbøl TB, Maeda K. The preclinical profile of Brexpiprazole: what is its clinical relevance for the treatment of psychiatric disorders? Expert Rev Neurother. 2015;15:1219-1229.
- Thakkar AM, Chhalotiya UK, Parekh N, Desai JV, Dalwadi HB, Shah DA. Quantification of Brexpiprazole in bulk and its pharmaceutical dosage form by UV-visible spectroscopic and SIAM RP-LC method. Austin Chromatogr. 2018;5:1050-1058.
- Sravani A, Naga Durga CH, Uppalapati D, Suneetha CH, Suresh P, Tirumaleswara RT, et al. Method development and validation for the estimation of Brexpiprazole in drug substance By Rp-Hplc method. Indo Am J Pharm Res. 2017;7:8560-8565.
- Nehal PB, Ashok BP, Rao MS, Amit JV, Nilesh KP, Ajay P. Development and validation of stability indicating assay method and characterization of degradation product for Brexpiprazole bulk by RP-HPLC. J Chem Pharm Res. 2018;10:55-66.
- Sowjanya B, Rambabu K. Development and validation for the simultaneous estimation of Brexpiprazole and fluoxetine in drug substance by RP-HPLC. Euro J Biomed Pharm Sci. 2018;5:411-417.
- Amit G, Rajendra P. Gradient high-performance liquid chromatography method for determination of related substances in Brexpiprazole API. Int J Dev Res. 2018;8:21416-21424.
- https://www.gmp-compliance.org/guidelines/gmp-guideline/ich-q2r1-validation-of-analytical-procedures-text-and-methodology
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q1b-photostability-testing-new-drug-substances-and-products
- Dey S, Patro SS, Suresh Babu N, Murthy NP, Panda KS. Development and validation of a stability-indicating RP-HPLC method for estimation of atazanavir sulfate in bulk. J Pharm Anal. 2017;7:134-140.
- http://www.pharmtech.com/fda-perspectives-scientific-considerations-forced-degradation-studies-anda-submissions?id=&sk=&date=&pageID=2
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q1ar2-stability-testing-new-drug-substances-and-products
Citation: Pulusu VS, Routhu KC, Chikkaswamy SSB (2019) Quantitative Determination of Brexpiprazole by RP-HPLC Method. Pharm Anal Acta 10:610. Doi: 10.24105/2153-2435.10.610
Copyright: © 2019 Pulusu VS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


