Indexed In
- Open J Gate
- Genamics JournalSeek
- CiteFactor
- Cosmos IF
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- ROAD
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 12, Issue 4
Post Pulmonary Tuberculosis: The Right Heart Story
Saira Jafri1, Nadia Jawad1*, Naseem Ahmed1, Nausheen Saifullah1 and Intisar Ahmad Siddiqui22College of Dentistry, Imam Abdulrahman bin Faisal University, Dammam, Saudi Arabia
Received: 30-Apr-2020 Published: 28-Jul-2020, DOI: 10.35248/0974-8369.20.12.466
Abstract
Background: We frequently come across patients whose debility had worsened even after completing pulmonary tuberculosis treatment. Data on the aftermath of tuberculosis from all over the world is insufficient.
Methods: A cross-sectional study was done retrospectively at Jinnah Postgraduate Medical Centre, Karachi, Pakistan. All the patients above 15 years of age who were correctly diagnosed and treated for pulmonary TB were taken from the last one year ’ s hospital files. Pulmonary artery systolic pressure was measured through trans-thoracic echocardiography.
Results: 88.9% patients had pulmonary hypertension of varying degrees; most of which fell in the mild and moderate category (44% and 42% respectively). Mean pulmonary artery pressure was 58.83 ± 18.45 mmHg. The estimated mean ± SD time of hospital-survival without PH was 16 ± 1.9 days whereas with PH it was 23.6 ± 1.9 days.
Conclusion: Since a substantial number of patients develop pulmonary hypertension after pulmonary tuberculosis, clinicians should thus maintain a high degree of suspicion with regard to pulmonary hypertension when following them.
Keywords
Pulmonary hypertension; Pulmonary tuberculosis; Echocardiography; Pulmonary artery pressure; Post TB
Abbreviations
PH: Pulmonary Hypertension; PTB: Pulmonary Tuberculosis; PASP: Pulmonary Artery Systolic Pressure; COPD: Chronic Obstructive Pulmonary Disease; PAP: Pulmonary Artery Pressure; WHO: World Health Organization; RHC: Right Heart Catheterization; ILD: Interstitial Lung Disease
Introduction
According to WHO, tuberculosis (TB) afflicted 10 million and killed 1.5 million people across the globe in 2018 [1]. Pulmonary Tuberculosis (PTB) incurs significant morbidity not only during the course of disease but also impairs quality of life in the years following treatment [2]. This may result from a number of factors, in part from airflow limitation [3] due to the permanent structural lung damage and subsequent hypoxia-related Pulmonary Hypertension (PH). Residual disability is very often overlooked when the impact of TB is described because more emphasis is placed on achieving cure/treatment completion. This impairment has been analyzed in several studies [4,5].
PTB as a cause of PH has been less reported. An Indian study highlighted that among patients with PH, 15% had a history of PTB [6]. Not many researches have looked at the presence of PH in patients treated for TB [6,7]. Since PH takes a toll on the quality of life of these patients, it is vital to address this when planning long-term care.
Considering that Pakistan is one of the high TB-burden countries, a large number of patients who present to us with respiratory symptoms have had TB in the past. From our population, data on the presence of PH in the aftermath of TB is scarce. We conducted a survey to determine the frequency of PH and its severity in treated TB patients now being admitted with dyspnea.
Materials and Methods
A cross-sectional study was done retrospectively at pulmonology ward, Jinnah Postgraduate Medical Centre, Karachi, Pakistan. All the patients were smear-negative at presentation. Our sample population comprised 90 patients above 15 years of age, admitted over past one year who were correctly diagnosed and treated for PTB at any point in their lives and had presented with worsening pulmonary symptoms.
Permission from the institutional ethical review committee was taken prior to the conduction of study. Demographic information and history about current and old symptoms was recorded along with basis, duration, treatment and outcome of past tuberculosis. Only those patients who were diagnosed and treated for TB according to WHO (and thus National TB Control Program) criteria were included [8]. The diagnosis of PH was made on the basis of composite echocardiographic criteria as it is a readily available, reliable, non-invasive modality when compared to right heart catheterization (RHC). The modified Bernoulli equation was used to determine the Pulmonary Artery Systolic Pressure (PASP) and the following conversions and cut-offs were employed [9] (Table 1).
Table 1: Pulmonary Artery Systolic Pressure (PASP) and conversions and cut-offs.
| Measured PASP | Estimated Mean PAP | Degree of PH |
|---|---|---|
| <40 mmHg | <25 mmHg | Normal |
| 40-60 mmHg | 25-40 mmHg | Mild |
| 60-90 mmHg | 41-55 mmHg | Moderate |
| >90 mmHg | >55 mmHg | Severe |
Data were analyzed on SPSS Version-20.0 (IBM Product, Chicago-USA). Severity of portal hypertension was the dependent outcome variable of the study. The independent factorial variables including gender, age groups, TB basis, duration of TB, smoking status, biomass exposure, comorbid, radiologic findings, arterial blood gases, in-hospital outcome and survival time were presented into frequencies and percentages. Numeric response variables like age, duration of TB and in-hospital survival time were presented into Mean ± Standard deviation. Analysis of variance (ANOVA) was performed to compare these variables among severity of PH groups. Pairwise size effects were evaluated by using Cohen’s d effect size estimators and Eta-squared for ANOVA test. Chisquare test was performed to compare proportions of demographic characteristics, clinical and radiological features of post TB patients and in-hospital outcome among different severities of PH in order to evaluate for any association. Binary logistic regression analysis was performed by taking presence or absence of PH as a binary dependent variable with a panel of 8 independent factorial variables (covariant) assuming normally distributed to evaluate the predictors of PH. Kaplan-Meier ’ s survival analysis was utilized to estimate in-hospital survival time. P-value ≤0.05 was considered statistically significant (Table 2).
Table 2: Relationship of demographic characteristics with the severity of post-tuberculosis pulmonary hypertension (PH).
| Factors | Severity of pulmonary hypertension (PH) | P-value | |||
|---|---|---|---|---|---|
| Mild (n=40) | Moderate (n=38) | Severe (n=2) | No PH (n=10) |
||
| Gender | |||||
| Male | 16 (40.0) | 12 (31.6) | 0 (0) | 5 (50.0) | 0.468 |
| Female | 24 (60.0) | 26 (68.4) | 2 (100) | 5 (50.0) | |
| Age (years) Mean ± S.D | 53.9 ± 17.8 | 49.3 ± 14.8 | 28.0 ± 5.6 | 47.4 ± 12.0 | 0.102 |
| Cohen’s d (effect size) | d1=0.385 | d2=0.133 | d3=-1.684 | È 2=0.263 | |
| Age ≤40 years | 8 (20) | 11 (28.9) | 2 (100) | 5 (50) | 0.154 |
| Age 41 - 60 years | 20 (50) | 19 (50.0) | 0 | 4 (40) | |
| Age >60 | 12 (30) | 8 (21.1) | 0 | 1 (10) | |
| TB Basis | |||||
| Sputum smear | 29 (72.5) | 24 (63.2) | 2 (100) | 4 (40.0) | 0.180 |
| X-ray/ clinical/ Unknown | 11 (27.5) | 14 (37.8) | 0 (0) | 6 (60.0) | |
| TB Years Ago (Mean ± S.D) | 10.9 ± 8.9 | 17.0 ± 12.1 | 2.0 ± 1.4 | 15.6 ± 12.0 | 0.035 |
| Cohen’s d (effect size) | d1=-0.492 | d2=0.116 | d3=-1.194 | È 2=0.095 | |
| TB 1 - 10 Year | 24 (60.0) | 14 (36.8) | 2 (100) | 4 (40) | 0.102 |
| TB 11 - 20 Years | 13 (32.5) | 12 (31.6) | 0 (0) | 4 (40) | |
| TB >20 years | 3 (7.5) | 12 (31.6) | 0 (0) | 2 (20) | |
| Smoking Status | |||||
| Non-smoker | 32 (80) | 32 (84.2) | 2 (100) | 6 (60.0) | 0.556 |
| Ex/current smoker | 8 (20) | 6 (15.8) | 0 (0) | 4 (40.0) | |
| Biomass Exposure | |||||
| Yes | 11 (27.5) | 8 (78.9) | 1 (50) | 1 (10) | 0.515 |
| No | 29 (72.5) | 30 (78.9) | 1 (50) | 9 (90) | |
Note: È 2=Eta squared for ANOVA test; Pairwise effect sizes: d1=(Mild vs. No PH); d2=(Moderate vs. No PH); d3=(Severe vs. No PH)
Results
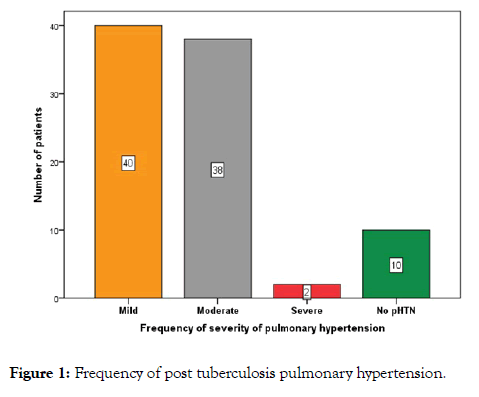
Out of 80 (88.9%) patients who had PH, 40 (44.4%) showed mild, 38 (42.2%) moderate and 2 (2.2%) showed severe degrees (Figure 1).
Figure 1: Frequency of post tuberculosis pulmonary hypertension.
On elaboration of population features, we saw a female preponderance 57 (63.3%) i.e. 1:1.7 male to female ratio. Mean age was 50.6 ± 16.2 years. Smear based diagnosis of TB was made in the maximum patients i.e. 59 (65.6%), followed by clinically diagnosed in 24 (27.8%) and radiologically diagnosed in 6 (6.7%). The time elapsed between previous treatment and inclusion was 13.2 ± 11 (ranging from 1 to 44) years. Majority of the patients had never smoked, precisely 72 (80%), 17 (18.8%) had smoked in the past while only 1 (1.1%) was currently a smoker. Biomass exposure was seen in 21 (23.3%) patients. Severity of PH was unrelated statistically to all these factors.
21 (23.3%) patients were suffering from other respiratory diseases simultaneously including smoker/non-smoker chronic obstructive pulmonary disease (COPD), some interstitial lung disease (ILD) or asthma. Co-morbidities including diabetes mellitus, hypertension or ischemic heart disease were present in 28 (31.1%). The radiological assessment identified 26 (28.9%) subjects with predominant fibrosis and 26 (28.9%) with predominant bronchiectasis whereas 35 (38.9%) had a mixed bronchiectasis-fibrosis picture. Only 2 patients (2.2%) had COPD radiologically and 1 (1.1) had pleural involvement. On arterial blood gases, 15 patients (16.7%) had type 1, 67 (74.4%) type 2 while in 8 (8.9%) there was no respiratory failure at all. Total 15 (16.6%) in-hospital mortalities occurred. Hospital stay of 81.1% patients was 1-2 weeks’ long and that of 17 (18.9%) patients was >2 weeks up to a maximum of 30 days. 10 days longer stay was in those with PH (mean hospital stay: 8.4 ± 5.40) (Table 3 and Figure 2).
Table 3: Relationship of clinical features and in-hospital outcome with the severity of post-tuberculosis pulmonary hypertension (PH).
| Factors | Severity of pulmonary hypertension (PH) | P-value | |||
|---|---|---|---|---|---|
| Mild (n=40) |
Moderate (n=38) | Severe (n=2) |
No PH (n=10) |
||
| Other Respiratory Disease* | |||||
| Yes | 9 (22.5) | 6 (15.8) | 0 (0) | 5 (50) | 0.114 |
| No | 31 (77.5) | 32 (84.2) | 2 (100) | 5 (50) | |
| Co-morbid† | |||||
| Yes | 15 (37.5) | 11 (28.9) | 0 (0) | 2 (20) | 0.508 |
| No | 25 (62.5) | 27 (71.1) | 2 (100) | 8 (80) | |
| Radiologic findings | |||||
| Predominant fibrosis | 10 (25.0) | 11 (28.9) | 0 (0) | 5 (50.0) | 0.371 |
| Predominant bronchiectasis | 11 (27.5) | 11 (28.9) | 2 (100) | 2 (20) | |
| Mixed bronchiectasis & fibrosis | 18 (45.0) | 15 (39.5) | 0 (0) | 2 (20) | |
| COPD/ Pleural involvement | 1 (2.5) | 1 (2.6) | 0 (0) | 1 (10) | |
| Arterial Blood Gases | |||||
| Type 1 | 7 (17.5) | 6 (15.8) | 1 (50) | 1 (10) | 0.659 |
| Type 2 | 29 (72.5) | 30 (78.9) | 1 (50) | 7 (70) | |
| No RF | 4 (10.0) | 2 (5.3) | 0 (0) | 2 (20) | |
| In-hospital Outcome | |||||
| Alive & discharged | 33 (82.5) | 32 (84.2) | 2 (100) | 8 (80) | 0.914 |
| Expired | 7 (17.5) | 6 (15.8) | 0 (0) | 2 (20) | |
| In-hospital Survival Time | 10.9 ± 6.4 | 8.8 ± 4.4 | 9.5 ± 3.5 | 9.4 ± 4.4 | 0.377 |
| Cohen’s d (effect size) | d1=0.247 | d2=-0.137 | d3=0.023 | È 2=0.035 | |
| Up to 1 week | 12 (30.0) | 19 (50.0) | 1 (50.0) | 3 (30.0) | 0.385 |
| Up to 2 weeks | 17 (42.5) | 14 (36.8) | 1 (50.0) | 6 (60.0) | |
| >2 weeks | 11 (27.5) | 5 (13.2) | 0 (0) | 1 (10.0) | |
Note: *smoker/non-smoker COPD, ILD, asthma, pleural diseases & others; †diabetes mellitus, hypertension, ischemic heart disease & others; È 2= Eta squared for ANOVA test; Pairwise effect sizes: d1=(Mild vs. No PH), d2=(Moderate vs. No PH), d3=(Severe vs. No PH)
Figure 2: Survival analysis for forecasting in-hospital survival time between patients with and without pulmonary hypertension (pHTN).
In binary logistic regression model, no predictor of post TB PH was found reveals none of the covariant effect on dependent outcome variable (Table 3). Survival analysis of the patients for 30 days ’ hospital stay related to PH has shown 2/10 (20%) mortalities in the patients without PH and 13/80 (16%) mortalities in patients with PH. The cumulative probability of survival is illustrated in Figure 2 and Table 4.
Table 4: Logistic regression analysis for prediction of factors association with post tuberculosis pulmonary hypertension.
| Factors | Post-TB pulmonary hypertension (pHTN) | Crude Odd ratio “OR” (95% C.I) | P-value | |
|---|---|---|---|---|
| pHTN (n=80) |
No pHTN (n=10) |
|||
| Gender (Male/ Female) | 52 (65.0) | 5 (50.0) | 0.74 (0.04-12.8) | 0.839 |
| Age (>60 years) | 20 (25.0) | 1 (10.0) | 0.91 (0.37-22.5) | 0.953 |
| Sputum smear TB basis | 55 (68.8) | 4 (40.0) | 7.72 (0.53-110.7) | 0.133 |
| TB years ago (>20 years) | 15 (18.8) | 2 (20.0) | 0.92 (0.18-4.80) | 0.819 |
| Smoking (Ex/ Current smoker) | 14 (17.5) | 4 (40.0) | 3.86 (0.24-61) | 0.338 |
| Biomass exposure | 20 (25.0) | 1 (10.0) | 0.41 (0.02-8.09) | 0.556 |
| Respiratory disease | 15 (18.8) | 5 (50.0) | 3.85 (0.46-32.5) | 0.215 |
| Co-morbid | 26 (32.5) | 2 (20.0) | 0.39 (0.04-3.66) | 0.407 |
| Radiologic presence of fibrosis | 54 (67.5) | 7 (70.0) | 0.89 (0.21-3.72) | 0.873 |
| In-hospital mortality | 13 (16.2) | 2 (20.0) | 24.0 (0.70-824) | 0.078 |
Discussion
PH is known to be a consequence of several chronic lung diseases, of which COPD, bronchiectasis and ILDs are well described [10]. The structural lung damage ensuing from tuberculosis infection may manifest as one of the above entities as documented in a study in Taiwan, where TB was identified as an independent risk factor for developing COPD [11]. In addition, tuberculosis may have an impact on the natural history of pre-existing lung conditions. We assessed our study cohort for the presence of underlying lung diseases and found that less than a quarter (23.3%) of them was already known to have COPD, asthma or ILD. So, the symptoms of most of our patients can be attributed to post-tuberculous sequelae alone. The study [2] by Vecino et al. on treated patients reported lower (12%) proportions of previously diagnosed lung disease. Our study included patients with respiratory co-morbids, which we consider a strength as it is a true depiction of population characteristics.
Tuberculosis causes permanently impaired lung function as early as 20 weeks after starting treatment as demonstrated by Vecino M et al. Spirometric abnormalities have been reported in majority of patients (52%, 86.8% [12]) by multiple studies, mostly showing an obstructive pattern. Moreover, Radovic M et al., noted that in patients in whom the initial impairment is usually restrictive, about 37% develop obstruction later depending on the extent of radiologic damage [13].
Several radiological manifestations have been described post pulmonary/extra pulmonary TB at parenchymal, airway, pleural, vascular as well as mediastinal levels [14]. In a systematic review of 37 studies deeming radiological findings post TB, the prevalence of bronchiectasis was the most followed by fibrosis [15]. Whereas in our patients, the majority exhibited a mixed fibro-bronchiectasis picture.
About a third (31.1%) of our study population had known ischemic heart disease or cardiovascular risk factors like diabetes and hypertension, which may contribute to mortality rates in the cohort. Lee C-H et al [11] reported diabetes as being the most common comorbid condition in TB patients developing COPD.
An overwhelming proportion of our patients were found to have PH. According to ESC/ERS guidelines, mild PH is common amid group 3 (chronic lung diseases) whereas severe PH is not [16]. Our findings were alike (44.4% mild vs. only 2.2% severe).
The development of PH in these cases is likely the result of chronic hypoxia from the structural and thus functional damage to lung tissue that occurs in the weeks or months following TB infection [17]. There is another mechanism described for COPD which may have a role after TB: the release of inflammatory mediators that cause vascular remodelling [18].
In our data time since TB treatment was 13.2 ± 11 years whereas in the study by Ahmed AE et al. it was 9.4 years and all the 14 subjects had PH [7]. Interestingly, in another study mean time between TB cure and echocardiographic assessment was just 8.9 months (range 2-45 months) plus 72 out of the 76 patients had PH [11]. Our data indicates that 10% of people had developed PH by one year time. Even more unanticipated and alarming is the fact pointed out by an Iranian study that as much as 9.7% people develop increased PASP during active TB infection [19].
Cigarette smoking is known to increase oxidative stress affecting coronary patency thus causing left ventricular dysfunction [20] so constituting a grand subgroup of PH [16,21] Additionally smoking unfavorably influences lung parenchyma and airways resulting in COPD hence triggering PH [22]. Smoke and biomass fuel exposure however did not contribute much to PH in our patients as almost two thirds of those who suffered were neither smokers nor did they report exposure to biomass. Therefore, the damage caused by exclusive tuberculosis seemed to be more causal, statistically insignificant though.
Severity grades of PH and their relationship with mortality has been studied a number of times and specifically 50% higher mortality was observed in those with PASP >60 mmHg compared with those with mild PASP which too had a high mortality of 23% during the follow-up [23]. Our in-hospital mortality was the highest among the mild PH category, followed by moderate PH.
In an Australian retrospective cohort assessing mortality in fibrotic lung diseases, they concluded that early (during one year) death is associated with PH rather than the severity of the underlying fibrotic lung disease [24]. Another survival analysis in systemic sclerosis showed that group 3 PH is worse in terms of survival than group 1 [25] mainly because of deteriorating oxygen saturations in ILD [26]. Bronchiectasis significantly affects right heart function thereby adding to mortality [27]. Our post TB population exhibits bronchiectasis besides fibrosis, higher PASPs and therefore inevitably longer hospital stays and deaths.
There are two limitations to our study. One limitation is that we included only patients who presented to us with symptoms severe enough to warrant admission (which implies advanced disease) and thus a substantial number of patients who were asymptomatic or had mild symptoms were not tested for PH. A broader study encompassing all cured/under treatment patients should be done to quantify the actual magnitude of PH after PTB treatment. Another limitation is that we have not accounted for infections like HIV which the patients may have and which can alter the course of PH.
Conclusion
To conclude, we put forward the incorporation of trans-thoracic echocardiography and spirometry in the standards of care during and after active TB infection so that any impairment in right heart function is timely diagnosed and managed.
REFERENCES
- World Health Organization. WHO Factsheet on Tuberculosis 17 Oct 2019.
- Vecino M, Pasipanodya JG, Slocum P, Bae S, Munguia G, Miller T, Fernandez M, Drewyer G, Weis SE. Evidence for chronic lung impairment in patients treated for pulmonary tuberculosis. Journal of infection and public health. 2011 Nov 1;4(5-6):244-52.
- Menezes AM, Hallal PC, Perez-Padilla R, Jardim JR, Muino A, Lopez MV, et al. Tuberculosis and airflow obstruction: evidence from the PLATINO study in Latin America. European Respiratory Journal. 2007 Dec 1;30(6):1180-5.
- Pasipanodya JG, Miller TL, Vecino M, Munguia G, Garmon R, Bae S, et al. Pulmonary impairment after tuberculosis. Chest. 2007 Jun 1;131(6):1817-24.
- Ralph AP, Kenangalem E, Waramori G, Pontororing GJ, Sandjaja, et al. (2013) High Morbidity during Treatment and Residual Pulmonary Disability in Pulmonary Tuberculosis: Under-Recognised Phenomena. PLoS ONE 8(11): e80302.
- Bhattacharyya P, Saha D, Bhattacherjee PD, Das SK, Bhattacharyya PP, Dey R. Tuberculosis associated pulmonary hypertension: The revelation of a clinical observation. Lung India 2016; 33:135-9.
- Ahmed AE, Ibrahim AS, Elshafie SM. Pulmonary hypertension in patients with treated pulmonary tuberculosis: analysis of 14 consecutive cases. Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine. 2011 Jan;5: CCRPM-S6437.
- National TB Control Program. Desk Guide for Doctors on Management of Tuberculosis. Revised 2019.
- Skinner GJ. Echocardiographic assessment of pulmonary arterial hypertension for pediatricians and neonatologists. Frontiers in pediatrics. 2017 Sep 4; 5:168.
- Seeger W, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension in chronic lung diseases. Journal of the American College of Cardiology. 2013 Dec 24;62(25 Supplement): D109-16.
- Lee C-H, Lee M-C, Lin H-H, Shu C-C, Wang J-Y, et al. (2012) Pulmonary Tuberculosis and Delay in Anti-Tuberculous Treatment Are Important Risk Factors for Chronic Obstructive Pulmonary Disease. PLoS ONE 7(5): e37978.
- Akkara SA, Shah AD, Adalja M, Akkara AG, Rathi A, Shah DN. Pulmonary tuberculosis: the day after. The International journal of tuberculosis and lung disease. 2013 Jun 1;17(6):810-3.
- Radovic M, Ristic L, Ciric Z. Changes in respiratory function impairment following the treatment of severe pulmonary tuberculosis - limitations for the underlying COPD detection. Int J Chron Obstruct Pulmon Dis. 2016; 11:1307-1316.
- Khan R, Malik NI, Razaque A. Imaging of Pulmonary Post-Tuberculosis Sequelae. Pakistan Journal of Medical Sciences. 2020 Jan;36(1): S75.
- Meghji J, Simpson H, Squire SB, Mortimer K. A systematic review of the prevalence and pattern of imaging defined post-TB lung disease. PLoS One. 2016;11(8).
- Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). European Respiratory Journal. 2015 Oct 1;46(4):903-75.
- Vyslouzil Z, Polák J, Widimský J, Suková M. Pathogenesis of pulmonary hypertension in tuberculosis. Czechoslovak Medicine. 1980 ;3(2):123-131.
- Saetta M, Baraldo S, Corbino L. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160: 711-717
- Marjani M, Baghaei P, Malekmohammad M, Tabarsi P, Sharif-Kashani B, Behzadnia N, et al. Effect of PH on outcome of PTB. Brazilian Journal of Infectious Diseases. 2014 Oct;18(5):487-90.
- Kamceva G, Arsova-Sarafinovska Z, Ruskovska T, Zdravkovska M, Kamceva-Panova L, Stikova E. Cigarette smoking and oxidative stress in patients with coronary artery disease. Open access Macedonian journal of medical sciences. 2016 Dec 15;4(4):636.
- Gerges C, Gerges M, Fesler P, Pistritto AM, Konowitz NP, Jakowitsch J, et al. In-depth haemodynamic phenotyping of PH due to left heart disease. European Respiratory Journal. 2018 May 1;51(5):1800067.
- Minai OA, Chaouat A, Adnot S. PH in COPD: epidemiology, significance, and management: pulmonary vascular disease: the global perspective. Chest. 2010 Jun 1;137(6):39S-51S.
- Strange G, Playford D, Stewart S, Deague JA, Nelson H, Kent A, Gabbay E. PH: prevalence and mortality in the Armadale echocardiography cohort. Heart. 2012 Dec 15;98(24):1805-11.
- Corte TJ, Wort SJ, Gatzoulis MA, Macdonald P, Hansell DM, Wells AU. Pulmonary vascular resistance predicts early mortality in patients with diffuse fibrotic lung disease and suspected PH. Thorax. 2009 Oct 1;64(10):883-8.
- Launay D, Humbert M, Berezne A, Cottin V, Allanore Y, Couderc LJ, et al. Clinical characteristics and survival in systemic sclerosis-related PH associated with interstitial lung disease. Chest. 2011 Oct 1;140(4):1016-24.
- Le Pavec J, Girgis RE, Lechtzin N, Mathai SC, Launay D, Hummers LK, et al. Systemic sclerosis-related PH associated with interstitial lung disease: impact of pulmonary arterial hypertension therapies. Arthritis & Rheumatism. 2011 Aug;63(8):2456-64.
- Öcal S, Portakal O, Öcal A, Demir AU, Topeli A, Çöplü L. Factors associated with PH and long-term survival in bronchiectasis subjects. Respiratory medicine. 2016 Oct 1;119:109-114.
Citation: Jafri S, Jawad N, Ahmed N, Saifullah N, Siddiqui IA (2020) Post Pulmonary Tuberculosis: The Right Heart Story. Biol Med (Aligarh) 12:466. doi: 10.35248/0974-8369.20.12.466
Copyright: © 2020 Jafri S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.