Indexed In
- Open J Gate
- Genamics JournalSeek
- Smithers Rapra
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 10, Issue 1
Pomegranate Peel Treated With Different Chemicals as Novel Biosorbent of Heavy Metals such as PB, CD, From Tanneries Effluents
Muhmmad Arslan Hameed##Equally contribution
Received: 10-Feb-2020 Published: 18-May-2020, DOI: 10.35248/2090-4568.20.10.194
Abstract
The effective removal of heavy metals from aqueous wastes is among the most important issues for many industrialized countries. Removal of cadmium (II) and lead (II) from aqueous solutions were studied using locally available and cost-effective pomegranate peel. Different chemical treatments were applied on it such as acid, alkali, formaldehyde and mixture treatment of formaldehyde and sulphuric acid. Batch adsorption experiments were performed in order to study the effect of biosorbent dose, time of contact and pH using untreated pomegranate peel powder for the purpose of optimizing the process of removing heavy metals. The optimum dose, contact time and pH required for maximum adsorption were found to be 5 g/100 cm3 of biosorbent dose, 5 hours contact time and pH= 3.0. The waste waters collected from different tanneries of Kasur were treated with different chemically treated biosorbents under these aforementioned optimum conditions. It was observed that acid treated pomegranate peel powder has better quality of adsorption of cadmium and lead from tannery waste water with percent removal 97 and 99 for cadmium and lead respectively.
Keywords
Adsorption; Biosorption; Pomegranate; Heavy metals
Introduction
The presence of heavy metals such as Pb, Cd, Cu, As, Ni etc. in the environment poses great health hazard. They body does not have a mechanism to remove these heavy metals unlike their organic counterpart which are readily removed [1]. Heavy metal contamination is a serious environmental problem which occurs due to contaminated water coming from many industries such as metal plating, tanneries, mining, painting, car radiator manufacturing as well as agricultural sources such as fertilizers and fungicides.
The removal of heavy metals from water becomes very important for this reason different technologies have been developed such as chemical precipitation [2], flotation [3], biosorption [4-6], electrolytic recovery, membrane separation [7], removal by adsorption on minerals [8,9] and activated carbon adsorption [10,11]. Despite their great efficiency, they remain elusive for small scale industries because of high cost of operation for example electrolytic recovery required great usage of electricity hence rendering them not suitable for large scale implementation. Chemical precipitation method relies on different chemical reagents and activated carbon is also dependent on quality of carbon [12]. It is the need of the hour to search for efficient and cost effective biosorbents. In recent year’s researchers have been busy investigating agricultural wastes as a new cost effective biosorbent. This include peat, wood, pine bark, banana pith, soybean, cotton seed hulls, peanut shells, hazelnut shells, rice husk, saw dust, wool, orange peel, compost and leaves [13].
Pomegranate fruit is used in fresh juice making and in processed forms used to make juice, jams and wine. Pomegranate peel (PP) is a byproduct of pomegranate juice industry and an inexpensive material. PP is rich in ellagitannins such as punicalagin and its isomers, as well as lesser amounts of punicalin, gallagic acid, ellagic acid and ellagic acid-glycosides [14]. PP is composed of several constituents such as polyohenols, ellagic tannis, gallic acid and ellagic acid [15]. In the present study the process is optimized by treating untreated PP with stock solutions of lead, cooper, cadmium and zinc. After the process has been optimized different chemically treated PP is treated with tanneries effluents collected from different tanneries.
Materials and Methods
Materials
A 1000 ppm solution of stock solution of lead nitrate and cadmium nitrate was prepared in distilled water respectively. From the stock solution of 1000 ppm of cadmium nitrate and lead nitrate 100 ppm solution was prepared respectively.
Biosorbent preparation
Pomegranate peel (PP) was collected from different juice shops located in Lahore. PP was washed with distilled water 20 times before stabilizing its pH from 1.93 to 7.0 and dried for 1 hour in an oven at 100°C. It was grounded in ball mill and sieved to particle size ranging from 0.2-0.5 mm. It was labeled as untreated PP. The weight of it was noted to be 1000 g. 200 g of untreated PP was first treated with 0.1 N solution of H2SO4 and soaked for 4 hours then dried in oven for about 1 hour at 100°C. It was stored and labeled as acid treated PP. About 200 g of untreated PP was treated with 0.1 N solution of NaOH and soaked for 4 hours and then dried in oven, it was stored and labeled as alkali treated PP. About 200g of untreated PP was treated 4% solution of formaldehyde and soaked in 4 hours and the dried in oven, it was stored and labeled as formaldehyde treated PP. 1 M solution of H2SO4 and 0.1M solution of formaldehyde were mixed and treated with untreated PP and soaked in for 4 hours. It was dried and then stored.
Adsorption experiments
Batch adsorption experiments were performed by treating untreated PP with 100 ppm solution of cadmium nitrate and lead nitrate respectively. Different parameters were investigated such as dosage effect, contact time and pH effect.
Results
Dosage effect
The result of this study is given in the following Table 1, Figures 1 and 2.
| Metal standard | Before Treatment (ppm) | Dosage | |||||
|---|---|---|---|---|---|---|---|
| 3 g | 5 g | 7 g | |||||
| After treatment (ppm) | % Removal | After treatment (ppm) | % Removal | After treatment (ppm) | % Removal | ||
| Cadmium | 100 | 25 | 75 | 19 | 81 | 19 | 81 |
| Lead | 100 | 18 | 82 | 12 | 88 | 12 | 88 |
Table 1: Biosorption of Cd and Pb using different dosages of biosorbent for fixed duration.
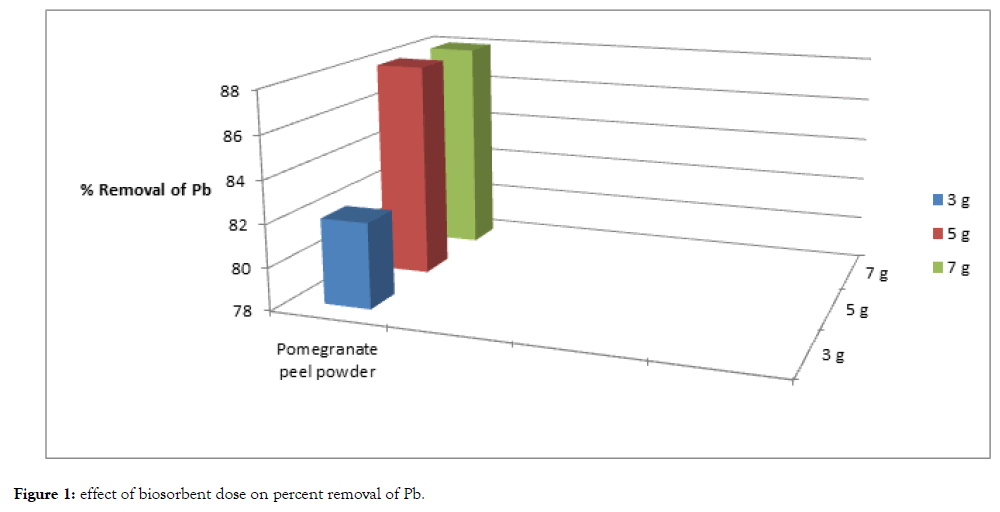
Figure 1: Effect of biosorbent dose on percent removal of Pb.
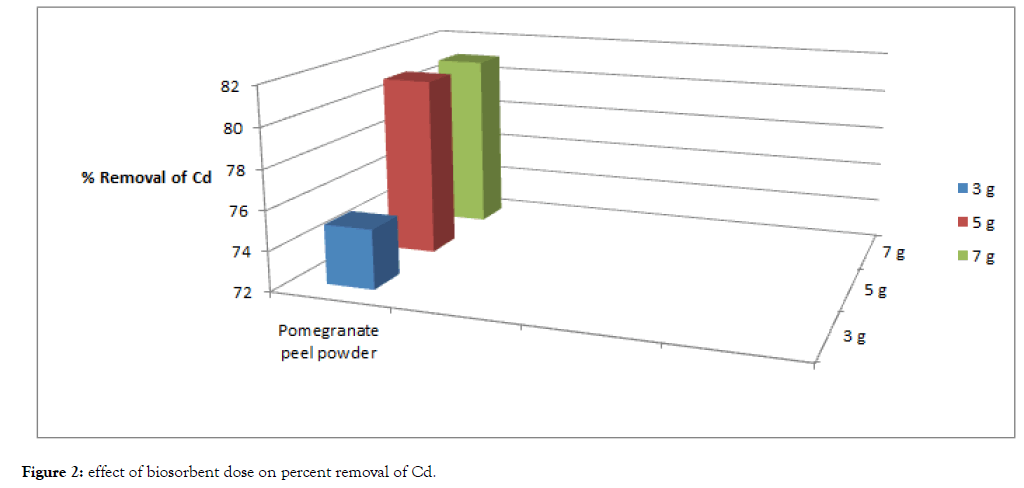
Figure 2: Effect of biosorbent dose on percent removal of Cd.
One of the parameters that strongly affect the sorption capacity is the dose or concentration of the biosorbent. With the fixed metal concentration, it can easily be inferred that the percent removal of metal ions increases with increasing weight of the biosorbent till a point reach where saturation of binding sites of biosorbent occurs. In this experiment with increase of dose the percent removal of lead and cadmium also increases till at higher dose with the increase of dose increase in percent removal of heavy metals does not takes place. The percent removal of cadmium and lead increased with increase of biosorption dose from 3 grams to 5 grams. However, with further increase of biosorbent dose i.e., from 5 grams to 7 grams saturation of binding sites of biosorbent occurred.
Contact time
From Table 2, Figure 3 and 4, The removal of cadmium and lead ions increases with increase in contact time i.e., from 3 hours to 5 hours and attains saturation in about 7 hours under condition of constant dose of biosorbent i.e., 5 grams of pomegranate peel powder.
| Metal Standard | Before Treatment (ppm) | Time duration | |||||
|---|---|---|---|---|---|---|---|
| 3 Hours | 5 Hours | 7 Hours | |||||
| After treatment (ppm) | % removal | After Treatment (ppm) | % removal | After treatment (ppm) | % removal | ||
| Cadmium | 100 | 19 | 81 | 15 | 85 | 15 | 85 |
| Lead | 100 | 12 | 88 | 10 | 90 | 10 | 90 |
Table 2: Effect of contact time adsorption while keeping dosage of biosorbent constant.
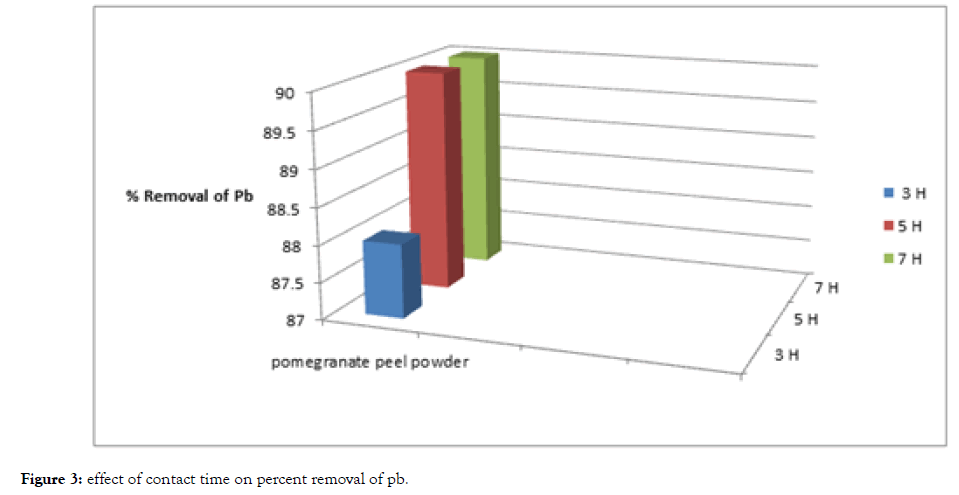
Figure 3: Effect of contact time on percent removal of pb.
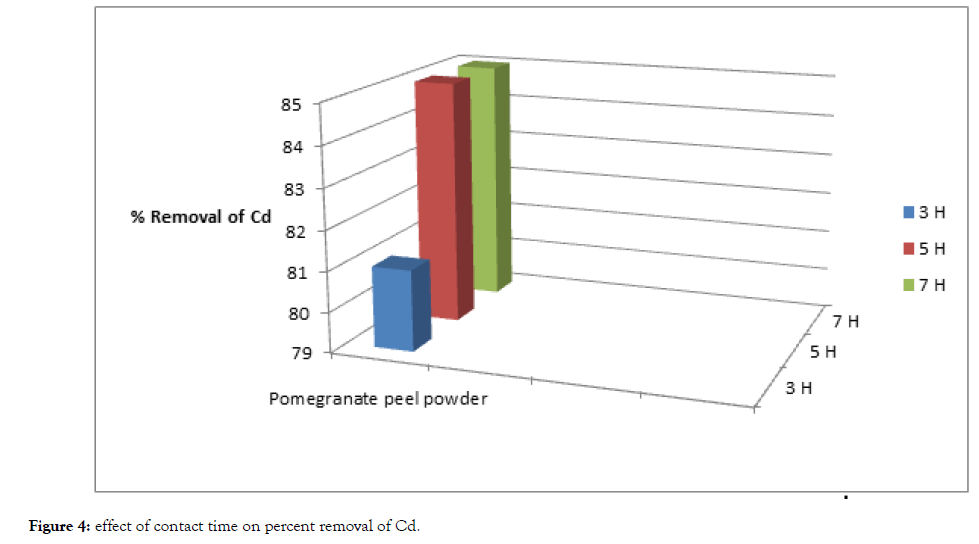
Figure 4: Effect of contact time on percent removal of Cd.
Basically, the removal of sorbate is rapid, but it gradually decreases with time until it reaches equilibrium. The two metals showed a fast rate of sorption during the first 3 to 5 hours of the removal becomes almost insignificant due to aquick exhaustion of the adsorption sites.
The rate of percent metal removal is higher in the beginning due to a larger surface area of the adsorbent being available for the adsorption of the metals.
The effect of pH
The pH of the solution has a significant impact on the biosorption of heavy metals, since it determines the surface charge of the adsorbent, the degree of ionization and speciation of the adsorbate and shown in Table 3, Figure 5 and 6.
| Metal Standard | Before Treatment (ppm) | pH | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 3.0 | 5.0 | 9.0 | 11.0 | ||||||
| After treatment (ppm) | % Removal | After Treatment (ppm) | % Removal | After treatment (ppm) | % Removal | After treatment (ppm) | % Removal | ||
| Cadmium | 100 | 11 | 89 | 14 | 86 | 20 | 80 | 22 | 78 |
| Lead | 100 | 8 | 92 | 11 | 89 | 17 | 83 | 20 | 80 |
Table 3: Effect of ph on biosorption under fixed dosage and contact time.
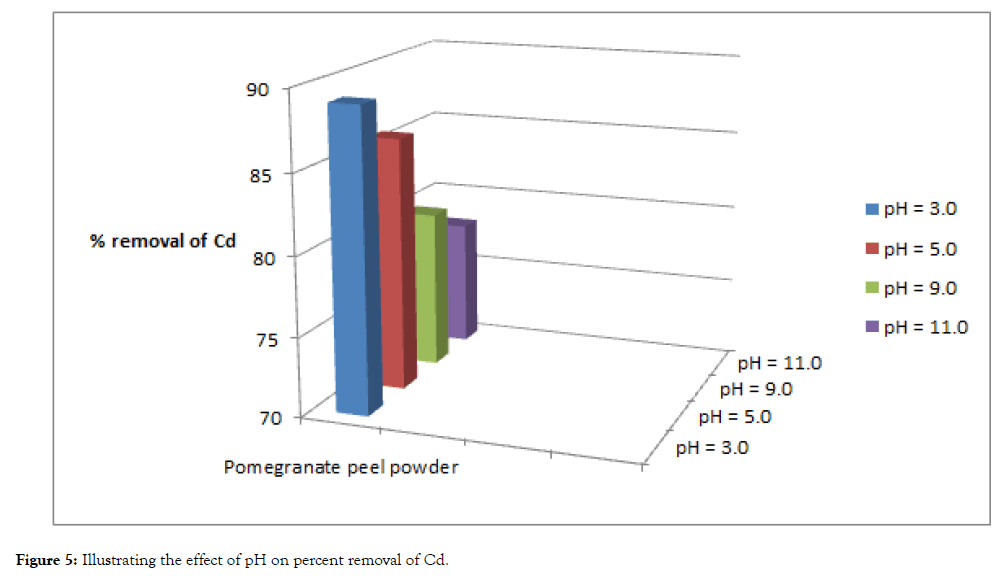
Figure 5: Illustrating the effect of pH on percent removal of Cd.
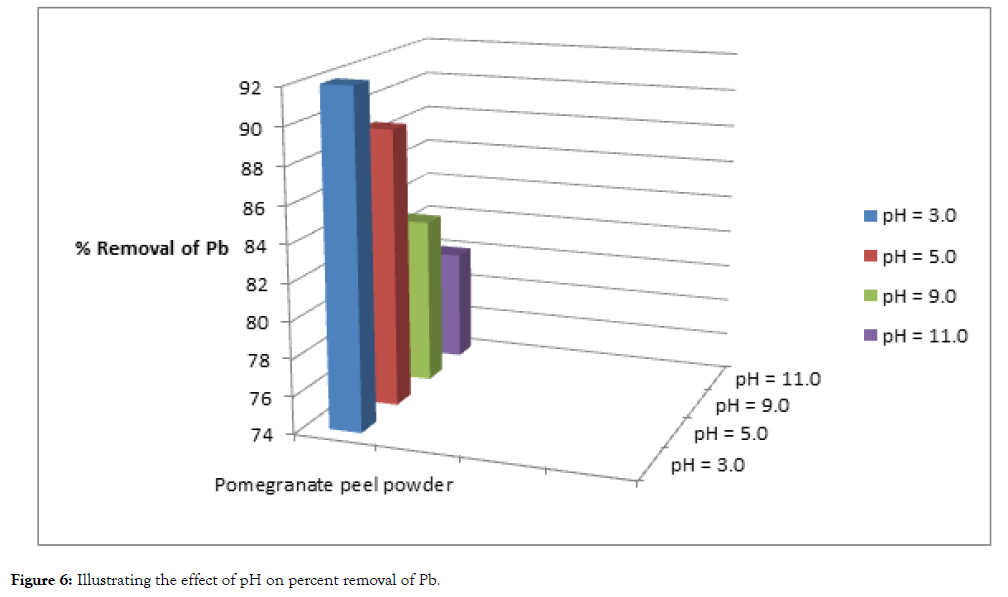
Figure 6: Illustrating the effect of pH on percent removal of Pb.
In order to establish the effect of pH on the biosorption of cadmium(II) and lead (II) ions, the batch equilibrium studies at different pH values were carried for the two metals respectively, under conditions of constant dose of biosorbent (5 grams) and contact time (5 hours). Adsorption was maximum at the most acidic pH, while uptake of heavy metals steadily decreased with the increase in pH values.
Treatment of chemical treated biosorbent with tannery waste water
Differently chemically treated pomegranate peel powder were treated with filtered waste water collected from different tanneries of Kasur under optimum conditions such as fixed dose i.e., 5 g, fixed contact time i.e., 5 hours and by adjusting the pH of filtered tannery waster to a pH= 3.0.
There are approximately 200 tanneries in Kasur and most of them are located in Niaz Nagar which is becoming the focal point of creating pollution and causing different diseases in neighboring areas. As far as tanneries are concerned, they are producing thousands of hazardous substances per day and these substances are being pumped into our environment.
The results of experiment are given below Table 4, Figures 7 and 8.
| Tanneries | Metal concentration before treatment | Metal concentration after treatment with acid treated pomegranate peel | Metal concentration after treatment with alkali treated pomegranate peel | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd (ppm) | Pb (ppm) | Cd (ppm) | % Removal | Pb (ppm) | % Removal | Cd (ppm) | % Removal | Pb (ppm) | % Removal | ||
| Ameer khan tannery | 20.38 | 22.62 | 0.42 | 97.93 | 0.20 | 99.11 | 2 | 90.18 | 5.5 | 75.68 | |
| Shan tannery | 25.71 | 20.93 | 0.70 | 97.27 | 0.18 | 99.14 | 2.5 | 90.27 | 5.2 | 75.15 | |
| Rehamn brother tannery | 23.95 | 26.35 | 0.70 | 97.07 | 0.18 | 99.31 | 2.3 | 90.39 | 6.5 | 75.33 | |
| Alkaram tannery | 20.54 | 29.48 | 0.50 | 97.56 | 0.20 | 99.32 | 2 | 90.26 | 7.3 | 75.23 | |
| Haseeb tannery | 27.35 | 27.39 | 0.60 | 97.80 | 0.20 | 99.26 | 2.5 | 90.85 | 6.8 | 75.17 | |
| Bismillah tannery | 21.42 | 25.57 | 0.60 | 97.19 | 0.18 | 99.29 | 2 | 90.66 | 6.2 | 75.75 | |
| Paradise tannery | 27.12 | 24.49 | 0.60 | 97.12 | 0.18 | 99.26 | 2.5 | 90.78 | 6 | 75.50 | |
| Yousuf welcome tannery | 26.78 | 32.65 | 0.60 | 97.75 | 0.18 | 99.44 | 2.5 | 90.66 | 8 | 75.49 | |
Table 4: Adsorption of Cd and Pb on treated biosorbents.
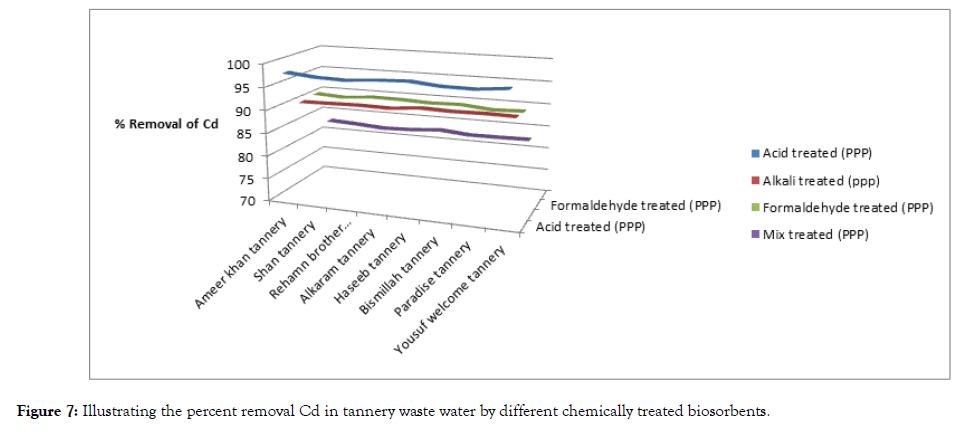
Figure 7: Illustrating the percent removal Cd in tannery waste water by different chemically treated biosorbents.
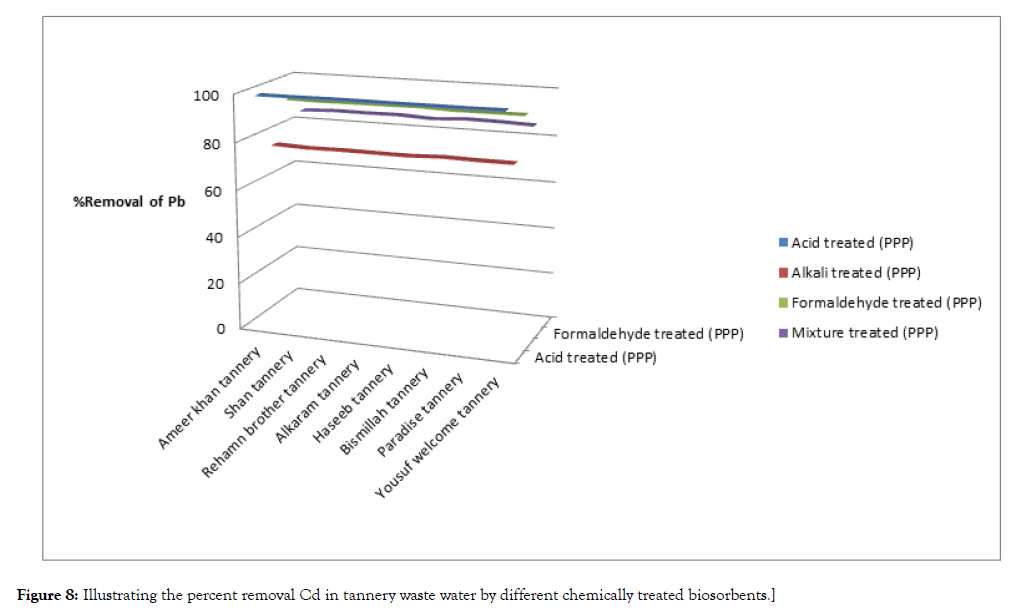
Figure 8: Illustrating the percent removal Cd in tannery waste water by different chemically treated biosorbents.
It is necessary to implement such methods that can create less pollution over there and people will remain safe from different diseases. Use of toxic chemicals is the main source of pollution especially in drinking water. The water is basically used for the purpose of cleaning the hides or skin. Tannery waste water is highly polluted in terms of heavy metals like cadmium and lead. Different chemically treated biosorbents were treated with 8 samples of waste water which were collected and sampled from 8 different tanneries of Kasur.
The percentage removal and concentration before treatments were determined by using atomic absorption spectrometer technique. The percentage removal can be calculated by using following formula.
 (1)
(1)
Where Co is initial concentration of heavy metal, Ci is final concentration of heavy metal.
Discussion
The effective removal of heavy metals from aqueous wastes is among the most important issues for many industrialized countries. Removal of cadmium (II) and lead (II) from aqueous solutions were studied using locally available and cost-effective pomegranate peel. Different chemical treatments were applied on it such as acid, alkali, formaldehyde and mixture treatment of formaldehyde and sulphuric acid. Batch adsorption experiments were performed in order to study the effect of biosorbent dose, time of contact and pH using untreated pomegranate peel powder for the purpose of optimizing the process of removing heavy metals. The optimum dose, contact time and pH required for maximum adsorption were found to be 5 g/100 cm3 of biosorbent dose, 5 hours contact time and pH= 3.0. The waste waters collected from different tanneries of Kasur were treated with different chemically treated biosorbents under these aforementioned optimum conditions. It was observed that acid treated pomegranate peel powder has better quality of adsorption of cadmium and lead from tannery waste water with percent removal 97 and 99 for cadmium and lead respectively. The better tendency of adsorption toward the lead is due to nature of pores of activated pomegranate. They surface acid treated pomegranate peel consist of such pores that favourable for sorption of large particles. The greatest biosorption of lead and cadmium were observed in wastewater of yousuf welcome tannery.
Conclusion
The observed tendency of biosorption for chemically treated pomegranate peel powder follow the following trend in terms of removal efficiency. Sulphuric acid treated pomegranate peel powder> formaldehyde treated pomegranate peel powder> Sodium hydroxide treated pomegranate peel powder> Sulphuric acid + formaldehyde mixture treated pomegranate. The optimum condition that was identified biosorbent dose of acid treated pomegranate peel 5 g/100 cm3, contact time of 5 hours and pH of 3. This novel activated biosorbent showed greater tendency for removal of lead as compared to cadmium owing to size of pores of that favoured biosorption of large particles.
REFERENCES
- Gupta VK, Gupta M, Sharma S. Process development for the removal of lead and chromium from aqueous solutions using red mud-an aluminium industry waste. Water Res. 2001; 35(5):1125-1134.
- Esalah JO, Weber ME, Vera JH. Removal of lead, cadmium and zinc from aqueous solutions by precipitation with sodium Di‐(n‐octyl) phosphinate. Can J Chem. Eng. 2000; 78(5):948-54.
- Zouboulis AI, Matis KA, Lanara BG, Loos-Neskovic C. Removal of cadmium from dilute solutions by hydroxyapatite. II. Flotation studies. Sep Sci Technol. 1997; 32(10):1755-67.
- Ho YS, Ng JCY, McKay G. Removal of lead (II) from effluents by sorption on peat using second-order kinetics. Sep Sci Technol. 2001; 36(2):241-61.
- Hall C, Wales DS, Keane MA. Copper removal from aqueous systems: biosorption by Pseudomonas syringae. Sep Sci Technol. 2001; 36(2):223-40.
- Sag Y, Akcael B, Kutsal T. Ternary biosorption equilibria of chromium (VI), copper (II), and cadmium (II) on Rhizopus arrhizus. Sep Sci Technol. 2002; 37(2):279-309.
- Canet L, Ilpide M, Seta P. Efficient facilitated transport of lead, cadmium, zinc, and silver across a flat-sheet-supported liquid membrane mediated by lasalocid A. Sep Sci Technol. 2002; 37(8):1851-60.
- Ahmad S, Khalid N, Daud M. Adsorption studies of lead on lateritic minerals from aqueous media. Sep Sci Technol. 2002; 37(2):343-62.
- Weirich DB, Hari R, Xue H, Behra P, Sigg L. Adsorption of Cu, Cd, and Ni on goethite in the presence of natural groundwater ligands. Environ Sci Technol. 2002; 36(3):328-36.
- Ravindran V, Stevens MR, Badriyha BN, Pirbazari M. Modeling the sorption of toxic metals on chelant‐impregnated adsorbent. AIChE J. 1999; 45(5):1135-46.
- Toles CA, Marshall WE. Copper ion removal by almond shell carbons and commercial carbons: batch and column studies. Sci Technol. 2002; 37(10):2369-83.
- Babel S, Kurniawan TA. Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard. Mater. 2003; 97(1-3):219-43.
- Pino GH, de Mesquita LM, Torem ML, Pinto GA. Biosorption of cadmium by green coconut shell powder. Miner Eng 2006; 19(5):380-7.
- Seeram N, Lee R, Hardy M, Heber D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep Purif Technol. 2005; 41(1):49-55.
- Nasr CB, Ayed N, Metche M. Quantitative determination of the polyphenolic content of pomegranate peel. Z Lebensm Unters Forsch. 1996; 203(4):374-8.
Citation: Hameed MA, Rehman S (2020) Pomegranate Peel Treated With Different Chemicals as Novel Biosorbent of Heavy Metals such as PB, CD, From Tanneries Effluents. J Adv Chem Eng 10:194. doi: 10.35248/2090-4568.20.10.194
Copyright: © 2020 Hameed MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.